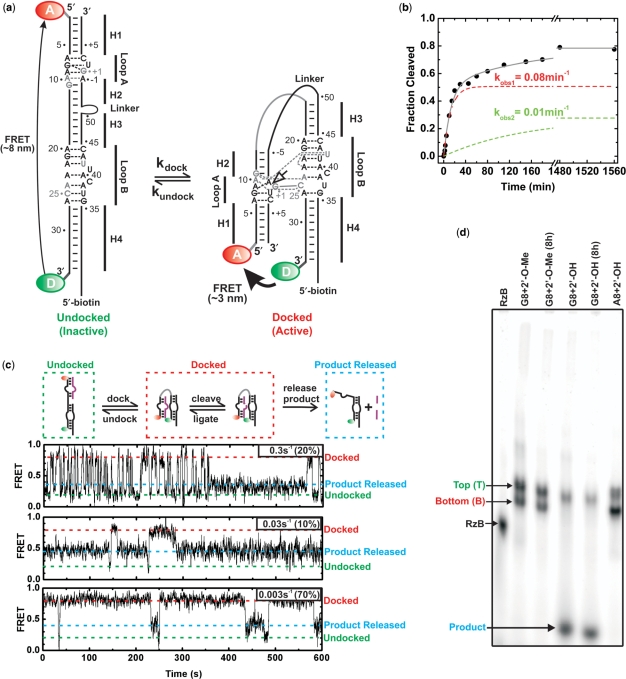
Figure 1.
Heterogeneity in folding and function of the hairpin ribozyme. (a) Secondary structure of the 2WJ hairpin ribozyme, composed of the RzA and RzB strands. The docked and undocked conformations of the ribozyme are shown with canonical and non-canonical base pairs indicated by solid and dashed lines, respectively. Nucleotides forming interdomain hydrogen bonds are shown in gray, the cleavage site is indicated by an open arrow. The ribozyme was labeled either with a domain terminal donor(D)–acceptor(A) fluorophore pair and a 5′-biotin, and/or with a 5′- or 3′-end 32P label. (b) Single-turnover cleavage time course of the 3′-32P-labeled ribozyme with a domain terminal donor–acceptor fluorophore pair and a 5′-biotin, as monitored by D-PAGE, was used to determine the indicated cleavage rate constants for the two phases of the reaction (12 mM MgCl2, pH 7.5, 25°C). The contribution of each phase is shown as a dashed line. (c) Heterogeneous undocking kinetics of the catalytically active, trans-cleaving (no Linker), t-2WJ hairpin ribozyme under multiple-turnover conditions. The docked, undocked and product released states are indicated by red, green and blue dashed lines, respectively. Three single molecule time trajectories demonstrating catalytic proficiency of distinct sub-populations (undocking rate constants and the fraction of molecules undocking with this rate constant are given in the upper right corner of each trajectory; see also Supplementary Figure S3). (d) EMSA separation of the 3′-32P-labeled ribozyme using a non-cleavable (2′-O-methyl) or cleavable (2′-OH) synthetic RzB strand, and inactive (transcribed with A8) or active (synthetic with fluorophores and G8) RzA strand, with and without an 8 h preincubation in native buffer. Two bands, termed T and B, are observed for ribozyme containing non-cleavable RzB (45% B) or inactive RzA (66% B). Cleavable ribozyme shows cleavage and subsequent product release for ∼60% of the material during electrophoresis (with <10% B band remaining). Preincubation of the ribozyme results in additional cleavage (>70%), consistent with the final cleavage extent in solution.