Scheme 3.
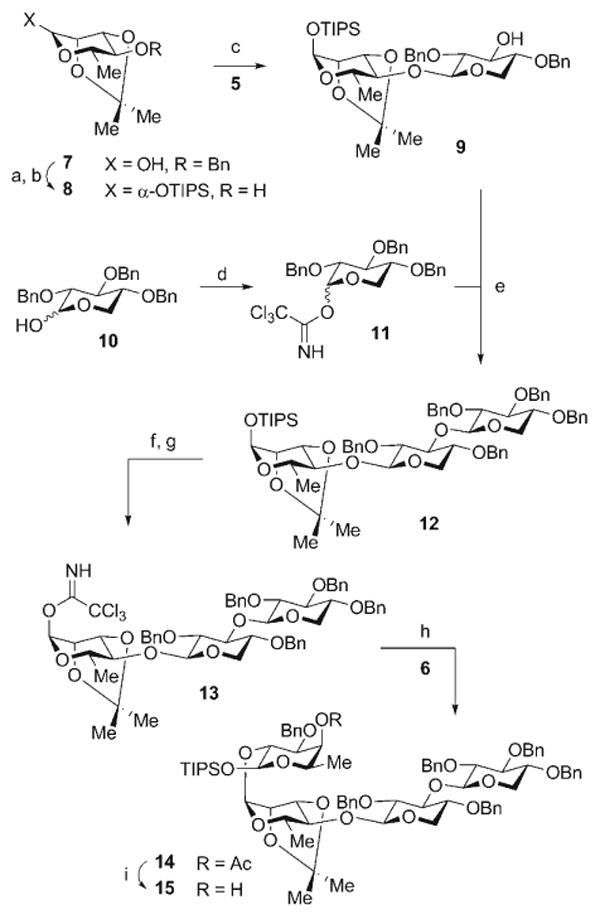
Reagents and conditions: a) TIPSOTf, 2,6-lutidine, CH2Cl2, 0→23 °C, 96%; b) Pd-C, H2, MeOH, 23 °C, 98%; c) 5, Ph2SO, Tf2O, TBP, CH2Cl2, −78°C; then 8, −78→35 °C, 75%; d) CCl3CN, NaH, CH2Cl2, 23°C, 82% (α/β 4:1); e) BF3·OEt2, CH2Cl2, −35°C, 77%; f) TBAF, THF, 0°C, >99%; g) CCl3CN, DBU, CH2Cl2, 0°C, 90%; h) 6, TMSOTf, Et2O, 0°C, 75%; i) DIBAL-H, CH2Cl2, −78°C, 83%. DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene, DIBAL-H = diisobutylaluminum hydride, TBAF = tetrabutylammonium fluoride, TBP = tri-tert-butylpyridine, Tf = trifluoromethanesulfonyl, TMS = trimethylsilyl.
