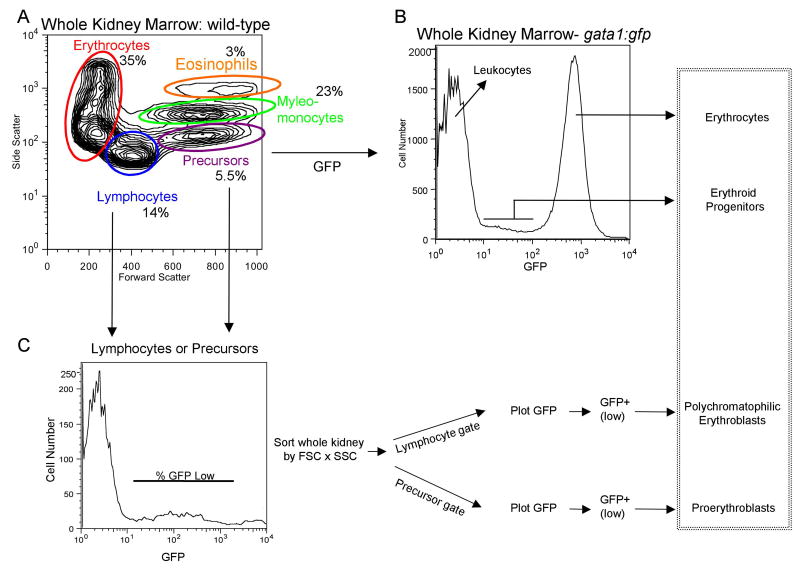
Fig. 1. Purification of hematopoietic cellular subsets and erythroid progenitors from adult zebrafish.
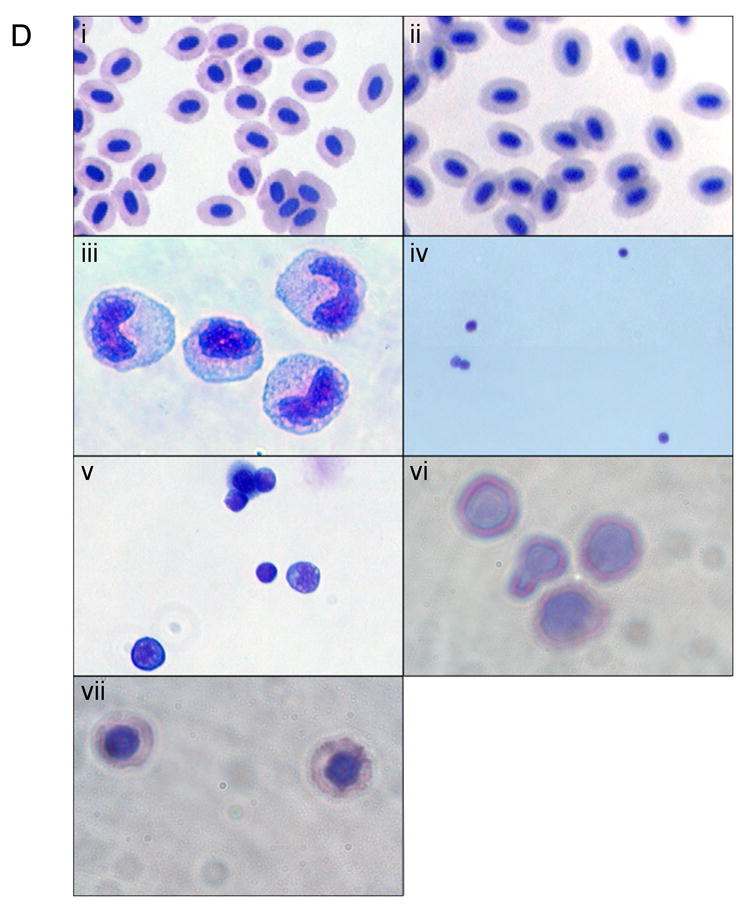
We applied general approaches described by Traver and colleagues [16,17] to purify cellular subsets from wild-type fish in our system, which are of distinct genetic backgrounds compared to those used in the published protocols. (A) Whole kidney marrow (WKM) was separated by flow cytometry (FACS). Dead cells were gated out by propidium iodide (PI) staining and the live cells analyzed by forward and side scatter profiles. The contour plot demonstrates that distinct populations can be identified: erythrocytes, myelo-monocytes, lymphocytes, and precursors. The average percentage of WKM for the populations are shown (n=11): erythrocytes: 35% ±9.2, lymphocytes: 14% ±3.1, precursors: 5.5% ±2.2, myelo-monocytes: 23% ±5.8. In addition, there is a minor eosinophil gate component that gates with higher relative side scatter from the myelo-monocyte group, approximately 3%. (B) FACS analysis of WKM from gata1:gfp reporter fish. A histogram representing relative GFP expression levels in cells from WKM is shown. Three distinct populations can be seen. A GFP negative peak, corresponding to the leukocytes, a bright GFP+ (high) peak corresponding to mature erythrocytes and a GFP+ low population that contains the erythroid progenitors. (C) The erythroid progenitors can be further discriminated using the gating strategy shown, similar to that described [17]. WKM was sorted by forward scatter (FSC), side scatter (SSC) and GFP, then contour plots of FSC vs. SSC were generated, and lymphocyte and precursor gates drawn. From each a GFP histogram was generated, and a GFP+ (low) gate drawn. The relative percent of GFP+ (low) cells was determined and used to track the erythroid progenitor populations. As shown in the box on the far right, these protocols allow accurate counts of adult erythroid cells at different developmental stages. (D) Shown are representative cytospin preparations of sorted cell types, as indicated. Cells were stained with Geimsa and May-Grünwald and photographed with a 100× oil objective. (i) peripheral erythrocytes, (ii) erythrocytes from WKM, (iii) myelo-monocytes, (iv) lymphocytes, (v) precursors, (vi) proerythroblasts, (vii) polychromatophilic erythroblasts.