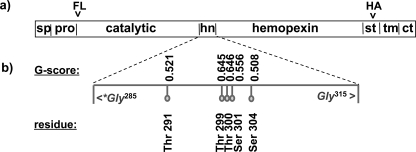
FIGURE 1.
Schematic domain model of MT1-MMP and tag localization. a, MT1-MMP has a distinct domain structure. In the constructs used for the present studies, the FLAG epitope tag (FL) was inserted between the furin cleavage site and the N terminus of the catalytic domain (ct). After the autocleavage of MT1-MMP and shedding of the catalytic domain the FLAG tag is thus released into the extracellular media. To ensure the capacity to detect the remaining membrane-associated portion of the cleaved protein, a HA tag (HA) was introduced in the stalk domain (st) that links the hemopexin domain to the transmembrane domain. This tag localization enables identification of most membrane-tethered MT1-MMP metabolites. b, glycosylation analysis. The protein sequence of MT1-MMP was analyzed with the NetNGlyc 1.0 Server and NetOGlyc 3.1 server for potential O- and N-linked glycosylation sites. No N-glycosylated residues were found. The prediction of O-glycosylated residues is based upon a trained neural network approach. If the score of the best general predictor, the G-score, is above the threshold of 0.5 then the residue is predicted to be glycosylated. All putative glycosylation sites are in the hinge domain (hn) of the protease (sp = signal peptide, pro = pro-domain, tm = transmembrane domain, ct = cytoplasmic tail).