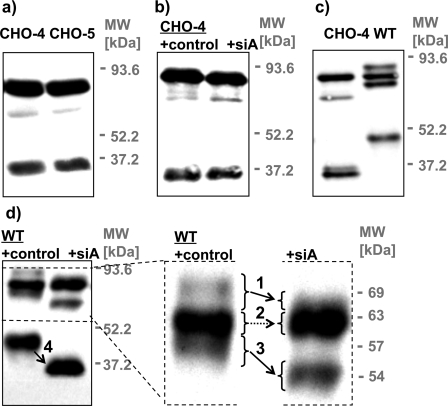
FIGURE 2.
Mutagenic elimination of glycosylation sites and enzymatic deglycosylation analysis of MT1-MMP. COS cells were transfected with MT1-MMP WT or constructs in which putative glycosylation sites were removed by mutagenesis (CHO). Cells were lysed 24 h after the transfection. Cell lysates were immunoblotted with monoclonal anti-HA Ab. a, mutants that are deficient in four (CHO-4 not including Ser304) or five (CHO-5 including Ser304) out of five putative O-glycosylation sites (compare Fig. 1b) showed no difference in electrophoretic mobility. b and d, cell lysates were incubated with sialidase A (siA) or solvent (control) prior to immunoblotting with monoclonal anti-HA Ab. b, CHO-4 displayed no shift in apparent molecular mass after sialidase A treatment, confirming that no additional sugars are attached at residue Ser304. c, expression of CHO-4 yields distinct bands of 63, 53, and two bands of ∼34 kDa, whereas expression of WT MT1-MMP results in the generation of bands of 69, 63, 57, and 44 kDa. d, glycosylated processed forms of MT1-MMP were in addition identified by a shift in their apparent molecular mass after sialidase A treatment (arrows 1, 3, and 4). This analysis confirms that the 63-kDa band (no shift, arrow 2) represents the only unglycosylated form of the WT protein.