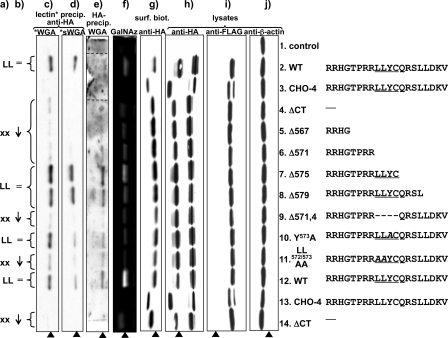
FIGURE 4.
Analysis of MT1-MMP constructs. COS cells were transfected with double (FLAG and HA)-tagged MT1-MMP constructs. Compare Fig. 1a for localization of the tags within the protein. a, presence (LL) or absence (xx) of the dileucine motif LL572 in the sequence of the cytoplasmic tail of the corresponding MT1-MMP protein construct. b, degree of glycosylation as detected by lectin binding and incorporation of azide modified galactosamine in comparison to the wild-type MT1-MMP protein. c and d, Western blot analysis of biotinylated wheat germ agglutinin (WGA)(c) or biotinylated, succinylated wheat germ agglutinin (sWGA)(d) precipitates. WGA binds GlcNAc and sialic acids, whereas sWGA binds only to GlcNAc. MT1-MMP was immunoblotted with monoclonal anti-HA Ab. e, MT1-MMP was precipitated with monoclonal anti-HA antibody, and Western blots were probed with biotinylated WGA and streptavidin-conjugated horseradish peroxidase (lanes 2 and 3 originate from the same blot, but their positions were shifted in the preparation of the figure to maintain the alignment of lanes throughout the entire panel). f, O-linked glycosides in MT1-MMP constructs were metabolically labeled in live COS cells with azide modified d-galactosamine (GalNAz) as a bioorthogonal tag. GalNAz was detected by covalent linking to TAMRA alkyne and imaging of TAMRA fluorescence in polyacrylamide gels after SDS-page analysis of proteins. g, transfected COS were cell-surface biotinylated. Biotinylated proteins were pulled down with streptavidin beads, and MT-MMP was detected with anti-HA Ab. As a control for protein expression and efficiency of protein loading and cell lysis, cell lysates were immunoblotted with monoclonal anti-HA Ab (h), monoclonal anti-FLAG Ab (i), and anti b-actin Ab (j)(arrowhead: O-glycosylated MT1-MMP self-cleavage product).