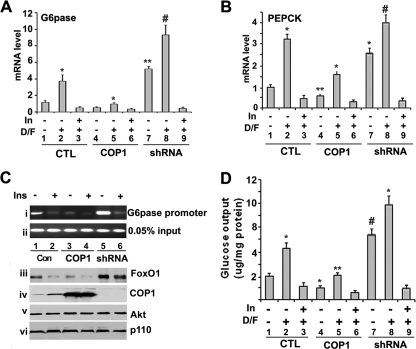
FIGURE 7.
COP1 regulates the expression of endogenous PEPCK and G6Pase gene. A, real time PCR analysis of endogenous G6Pase and PEPCK genes expression. Fao cells were infected with GFP (CTL) or COP1- or COP1-shRNA (shRNA)-expressing adenoviruses. 30 h post-infection the cells were serum-starved overnight followed by 4 h of treatment with either DMSO, 0.5 μm dexamethasone, 2.5 μm forskolin (D/F), or 0.5 μm dexamethasone, 0.5 μm forskolin, 100 nm insulin (In). RNA was prepared for real time-RT-PCR to detect G6Pase gene expression. B, same as A, except that here we assessed expression of PEPCK. C, chromatin immunoprecipitation assay to show FoxO1 binding to G6Pase promoter. Fao cells in 15-cm dishes were processed as in A except for the chromatin immunoprecipitation assay. The presence of the G6Pase promoter (panel i) in the chromatin immunoprecipitate of anti-FoxO1 and in the chromatin preparation before immunoprecipitation (input, panel ii)) was then assayed using PCR as described under “Experimental Procedures.” In addition, a portion of cells were used to prepare the total cell lysates for Western blotting with anti-FoxO1 (panel iii), anti-COP1 (panel iv), anti-Akt (panel v), and anti-p110γ (panel vi) antibodies. D, glucose output assay. The same cells as in A were serum-deprived overnight, and the medium was replaced with glucose-free, phenol-free medium. After 4 h of treatment with the indicated reagents, the medium was collected and subjected to glucose assay with Sigma glucose assay reagents, and d-glucose served as a standard for the assay. The experiments were carried out in triplicate, and the amount of glucose in the medium was normalized to levels of total cellular protein. In all the experiments, each bar represents the mean ± S.D. (n = 3). The p values were related to the control conditions. *, p < 0.015; **, p < 0.005; #, p < 0.011.