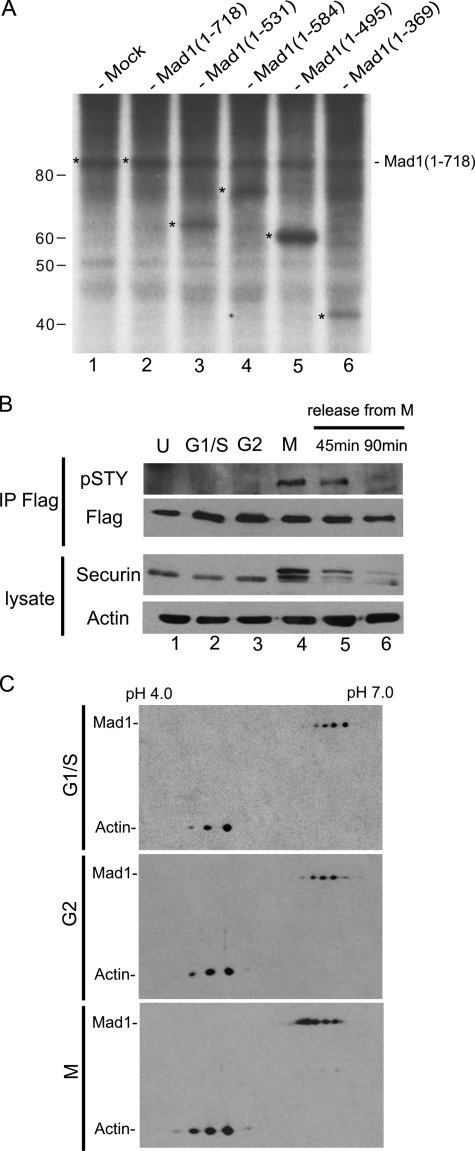
FIGURE 1.
Cell-cycle dependent phosphorylation of Mad1. A, phosphorylation of human cell endogenous Mad1 (lane 1), and Mad1 in cells transfected with plasmid that express Mad1 wild type (WT, 1-718; lane 2) or the indicated Mad1 mutants (amino acids 1-531, 1-584, 1-495, and 1-369; lanes 3-6). Phosphorylated proteins were biosynthetically labeled with [32P]orthophosphate. Mad1 WT and the indicated mutant proteins were immunoprecipitated using a mixture of two antibodies that recognize the N and C terminus of Mad1, respectively. The immunoprecipitates were analyzed by SDS-PAGE followed by autoradiography. 32P-Incorporated bands of Mad1 WT and mutants are denoted by the asterisk. B, Mad1 was immunoprecipitated from a HeLa cell line stably expressing a FLAG-Mad1 transgene using anti-FLAG-agarose beads. The immunoprecipitates were electrophoresed and then immunoblotted using an αpSTY antibody that specifically recognizes a phosphorylated serine/threonine/or tyrosine residue. Immunoblotting using anti-FLAG (Flag) verified the immunoprecipitation of FLAG-Mad1. Cells were asynchronous (U), synchronized in G1/S, G2, M (nocodazole-arrested), or were released from nocodazole for 45 or 90 min, as indicated. Immunoblotting of securin was used to reflect cell cycle synchronization status, and blotting for actin was used to monitor equal sample loading. C, two-dimensional gel electrophoresis followed by immunoblotting of cell endogenous Mad1 from G1/S, G2, and M phase-enriched HeLa cells. Actin was used to align the positioning of the Mad1 two-dimensional spots. Compared with G1/S- and G2-synchronized cells, more Mad1 two-dimensional spots in M phase are shifted toward the cathode consistent with hyperphosphorylation and lowered isoelectric points (pI).