SYNOPSIS
Objectives
The incidence of hepatocellular carcinoma (HCC) in the United States has increased dramatically over the last two decades, largely because of an increase in the number of people with advanced hepatitis C virus (HCV) infection. U.S. prisoners are at high risk for HCC, given their elevated rates of HCV infection, comorbid hepatitis B virus (HBV) infection, and alcoholic liver disease. The purpose of our study was to examine the prevalence and mortality of HCC in the nation's largest state prison system.
Methods
The study population consisted of 325,477 male Texas Department of Criminal Justice (TDCJ) inmates who were incarcerated between January 1, 2003, and July 31, 2006. Information on medical conditions and demographic characteristics was obtained from an institution-wide medical information system.
Results
During the 3.5-year study period, 176 male TDCJ inmates (54 per 100,000) were diagnosed with HCC and 108 (33 per 100,000) died as a result of HCC. Inmates who were Hispanic, older, and infected with HCV, HBV, or human immunodeficiency virus had elevated rates of both HCC prevalence and mortality. After adjusting for all study covariates, HCC prevalence, but not mortality, was modestly elevated among inmates with diabetes.
Conclusions
Our study showed that the Texas male prison population had a sevenfold higher prevalence of HCC than the general U.S. male population and a fourfold higher death rate from HCC. These findings likely reflect the high concentration of HCC-related risk factors, particularly HCV, among prisoners.
The incidence of hepatocellular carcinoma (HCC) in the United States has increased rapidly over the last two decades, largely as a result of an increase in the number of people with cirrhosis from hepatitis C virus (HCV) infection.1–4 The HCV epidemic in the U.S. is estimated to have started in the 1960s and peaked in the 1980s, during which HCV risk factors such as injection drug use, needle sharing, and unprotected high-risk sexual behavior increased.5 These risk factors, which are particularly concentrated among incarcerated populations, have resulted in the U.S. correctional system bearing a disproportionate burden of the nation's HCV epidemic. Compared with the general U.S. population, the nation's prison population is reported to have a 10- to 20-fold higher rate of HCV infection6–10 and a threefold higher mortality rate from end-stage liver disease.11 Additionally, several studies have found that prison inmates exhibit elevated rates of hepatitis B virus (HBV) infection7,8 and a history of alcohol abuse,12,13 both of which are risk factors for HCC. To our knowledge, no published reports describing the epidemiology of HCC in correctional populations are available. The purpose of this study was to examine HCC prevalence and mortality in the nation's largest state prison system.
METHODS
This investigation was a prevalence study of 325,477 male inmates incarcerated in the Texas Department of Criminal Justice (TDCJ) prison system for any duration between January 1, 2003, and July 31, 2006. All TDCJ inmates—both male and female—were initially included in this investigation. However, after determining that there was only a single case of HCC among the female inmate population, we restricted the overall analysis to males. The study was reviewed and approved by the University of Texas Medical Branch (UTMB) Institutional Review Board.
Data sources
The primary data source for this study was an electronic database that contains the medical records of all TDCJ inmates. UTMB provides health-care services for about 80% of the state prison population; Texas Tech University Health Sciences Center (TTUHSC) serves the remainder of the population. All TDCJ inmates undergo medical and mental health examinations at the time of intake. This evaluation lasts approximately 60 minutes and consists of a detailed medical and mental health history, a comprehensive physical examination, and a number of diagnostic procedures, including a rapid plasma reagin test and Mantoux tuberculosis skin test. Additionally, all inmates who have a family history of diabetes or other risk factors for the disease are screened via a fasting glucose test.
Medical conditions diagnosed at the time of the initial evaluation and/or subsequent medical encounters that occur during the inmate's incarceration are classified according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) coding system and recorded in the inmate's electronic medical record (EMR). An inmate was considered to have a diagnosis of HCC if his or her EMR contained an ICD-9-CM code of 155.0 (malignant neoplasm of liver, primary). The UTMB cancer registry was used as a secondary source to identify TDCJ inmates with HCC.
About 90% to 95% of TDCJ inmates with HCC were treated at UTMB; the remainder were treated at TTUHSC. Although hospitalization and cancer registry records from TTUHSC were not available for this study, the vast majority of patients treated at TTUHSC would likely have had at least one visit at UTMB and would, therefore, have been listed in either the UTMB cancer registry or hospital records. Also, the majority of these patients would have had information on their condition entered into the EMR. Of all cases of HCC that were identified in the study population, 85% were identified in both the EMR and the UTMB cancer registry databases, 14% were identified exclusively in the EMR database, and 1% were identified exclusively in the UTMB cancer registry database. All cases of mortality related to HCC were verified from mortality records (which include cause of death information) that are maintained on all TDCJ inmates who die during incarceration.
Human immunodeficiency virus (HIV) and hepatitis screening
All TDCJ inmates are offered serological screening for HIV infection during their incarceration and at the time of their release. Approximately 90% of the study cohort underwent HIV screening during the follow-up period. Inmates who test positive for HIV are also screened for HCV and HBV infection within two to four weeks after confirmation of their HIV-seropositive status. TDCJ does not have a universal HCV and HBV screening policy for inmates who are not HIV-infected. A decision to screen for HCV and HBV is based on the inmate's self-reported risk behaviors (e.g., intravenous drug use or multiple sex partners), the inmate's self-reported medical history, acute illness consistent with hepatitis, presence of elevated transaminase levels, or the inmate's request for hepatitis screening. Using serological testing, HCV and HBV positivity were determined as follows. A diagnosis of HCV was defined as the presence of immunoglobulin G antibody to HCV. Likewise, a diagnosis of HBV was defined as the presence of hepatitis B surface antigen. These tests relied on serum and plasma samples from the study cohort using VITROS® immunodiagnostic products (Ortho-Clinical Diagnostics, Inc., Rochester, New York).
Statistical analysis
All statistical analyses were performed using the GENMOD procedure in SAS®.14 Negative binomial regression analysis was used to examine differences in HCC prevalence and mortality across the subgroups and to calculate adjusted prevalence rate ratios and corresponding 95% confidence intervals (CIs). Negative binomial regression analyses adjust for skewed distributions and are thus favored when studying relatively rare events. After exponentiation, the regression coefficients were interpreted in terms of relative rates.
RESULTS
HCC prevalence
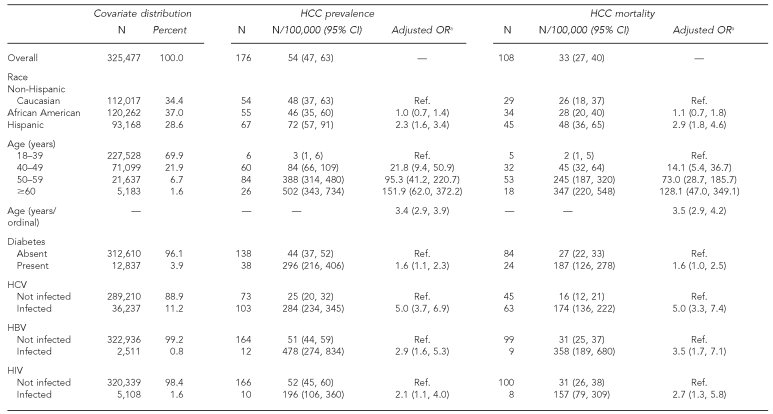
During the 3.5-year study period, 176 male inmates (54 per 100,000) were diagnosed with HCC, and 108 deaths (33 per 100,000) were attributed to HCC. Prevalence and mortality rates (per 100,000) for HCC are reported according to demographic factors in the Table. Overall, the prevalence of HCC was higher among males (54 per 100,000) than females (two per 100,000). Because only one female inmate had HCC, the majority of the analyses were restricted to males.
Table. HCC prevalence and mortality among Texas Department of Criminal Justice male inmates, January 2003 to July 2006.
aAll covariates are adjusted for race, age, diabetes, HCV, HBV, and HIV.
HCC = hepatocellular carcinoma
CI = confidence interval
OR = odds ratio
Ref. = reference group
HCV = hepatitis C virus
HBV = hepatitis B virus
HIV = human immunodeficiency virus
Among males, the prevalence was higher among Hispanic (72 per 100,000) than African American (46 per 100,000) or non-Hispanic Caucasian (48 per 100,000) inmates. HCC prevalence increased in a stepwise fashion according to age (three per 100,000 for age 18–39 years; 84 per 100,000 for age 40–49 years; 388 per 100,000 for age 50–59 years; and 502 per 100,000 for age ≥60 years). The prevalence of HCC was also elevated among inmate cohorts with diabetes (296 per 100,000), HCV infection (284 per 100,000), HBV infection (478 per 100,000), and HIV infection (196 per 100,000). After simultaneously adjusting for all study covariates (Table), an elevated risk of HCC persisted for inmates who were Hispanic (odds ratio [OR] = 2.3, 95% CI 1.6, 3.4) and older (OR=3.4, 95% CI 2.9, 3.9). The risk of HCC also remained significantly elevated for inmates with diabetes (OR=1.6, 95% CI 1.1, 2.3), HCV infection (OR=5.0, 95% CI 3.7, 6.9), HBV infection (OR=2.9, 95% CI 1.6, 5.3), and HIV infection (OR=2.1, 95% CI 1.1, 4.0). There were no statistically significant interaction effects among all combinations of HCV, HBV, HIV, and diabetes.
HCC mortality
Similar to HCC prevalence, HCC mortality (Table) was higher among males (33 per 100,000) than females (two per 100,000). Once again, after restricting the analysis to male inmates, the prevalence of HCC mortality was higher among Hispanic (48 per 100,000) than African American (28 per 100,000) or non-Hispanic Caucasian (26 per 100,000) inmates, and increased in a stepwise fashion according to age (two per 100,000 for ages 18–39 years; 45 per 100,000 for ages 40–49 years; 245 per 100,000 for ages 50–59 years; and 347 per 100,000 for ages ≥60 years). HCC mortality was also elevated in male inmates with diabetes (187 per 100,000) and all three cohorts with viral infections: HCV infection (174 per 100,000), HBV infection (358 per 100,000), and HIV infection (157 per 100,000).
The multivariate model for HCC mortality (Table) indicated that an elevated risk persisted for inmates who were Hispanic (OR=2.9, 95% CI 1.8, 4.6) and older (OR=3.5, 95% CI 2.9, 4.2), and for those who had HCV monoinfection (OR=5.0, 95% CI 3.3, 7.4), HBV monoinfection (OR=3.5, 95% CI 1.7, 7.1), and HIV infection (OR=2.7, 95% CI 1.3, 5.8). There were no statistically significant interactions among any of the four diseases under study (i.e., diabetes, HCV, HBV, and HIV).
DISCUSSION
Although numerous studies have documented that a large proportion of U.S. prisoners have major risk factors for HCC, including HCV and HBV infection as well as alcoholic liver disease, little information about the epidemiology of HCC in correctional populations is available. Our study showed that the prevalence of HCC among males in the nation's largest state prison is approximately seven times higher (54 per 100,000) than the rate in the general U.S. population (seven per 100,000), and that the HCC mortality rate is four times higher (33 per 100,000) in the prison population than in the general population (7.4 per 100,000).15
It is important to note, however, that because the dates of disease diagnosis, incarceration, and release were not available for a substantial proportion of the study population, our investigation relied on estimates of HCC prevalence per 100,000 people, as compared with the aforementioned studies' estimates of incidence of HCC per 100,000 people. It is possible that the short incarceration periods of many inmates resulted in a lower rate of HCC ascertainment. Inmates with short prison sentences contributed sufficiently to the assessment of the at-risk population (i.e., the denominator), but may have had too short a period of incarceration to have sufficiently contributed to the number of cases (i.e., the numerator). Such bias is particularly problematic when evaluating diseases that exhibit a rapid progression to mortality. It is important to consider, therefore, that the gradient between prison and general population estimates may be underestimated in this investigation.
The epidemiology of HCC in the Texas prison population, like that of the general U.S. population,4 is characterized by substantial variation across demographic characteristics. We found that the prevalence of HCC was 16 times higher among male inmates than among female inmates (OR=16.5, 95% CI 2.3, 117.7) and that the HCC mortality rate was 60 times higher in males than in females (OR=9.5, 95% CI 1.4, 7.4). These findings should be interpreted with some caution given the single HCC case on which the rates for female inmates were based. Nevertheless, the gender difference in HCC prevalence observed in our study is substantially greater than for the general U.S. population, where age-adjusted rates of HCC are only three to four times higher for men.1–4,16 This finding is particularly surprising given that the gender-HCV risk gradient in incarcerated populations is the opposite of that reported in the general population,6 as females are more likely than males to be incarcerated for offenses that are also risk factors for HCV infection, such as injection drug use, crack/cocaine use, and prostitution.17 Possible reasons for the higher rates of HCC among males in the general U.S. population include higher levels of testosterone, higher body mass index values, and higher rates of HCV infection and other liver disease among men.18 However, it is not clear which, if any, of these factors would differentially affect incarcerated populations.
Our finding that HCC prevalence and mortality increased in a stepwise fashion according to age is generally consistent with studies of nonincarcerated populations.1,4,19 Epidemiologic studies of the general U.S. population indicate that while HCC rarely occurs before the age of 40, the rate steadily increases afterward, generally reaching its peak between the ages of 70 and 75.4
Additionally, consistent with previous findings for the general U.S. population,18 our study showed that Hispanic inmates had elevated rates of both HCC prevalence and mortality compared with non-Hispanic Caucasian inmates. However, results from a recent study using National Health and Nutrition Examination Survey data collected from 1999 to 2002 indicate that the overall prevalence of HCV infection was not elevated among Mexican Americans,20 a finding consistent with our HCV seroprevalence study of offenders entering the Texas prison system in 1999.6 These studies raise the question of whether Hispanic inmates with HCV infection may be, for some reason, more likely to develop HCC. Recent evidence suggests that diabetes may act synergistically with HBV and HCV to induce HCC.16,21 It is possible that diabetes and related metabolic disorders, which have reached epidemic proportions among Mexican Americans,22,23 play a significant role in the development of HCC among this population. Although our analyses showed no statistically significant interaction effects between diabetes and either HCV or HBV overall or in any of the race/ethnicity subgroups, it is important to acknowledge that the underestimation of true HCV and HBV prevalence in the TDCJ may have affected the validity of these effect estimates.
Finally, it should be noted that in contrast with studies of the general U.S. population that have reported a twofold greater risk of HCC among African American males (similar to that of Hispanic males) compared with non-Hispanic Caucasian inmates,18 our study showed that African American male inmates were at no greater risk for either developing HCC or dying from it than non-Hispanic Caucasian males.
Our finding that HCV and HBV infection were both associated with elevated HCC prevalence and mortality is consistent with observations in the general population.1,3,18,19 However, the fact that we found no statistically significant interactions between HIV and either HCV or HBV is somewhat surprising, as numerous investigators have reported that coinfection with HIV and HCV increases the risk of developing cirrhosis and accelerates progression to cirrhosis.24–29 In view of the paucity of published evidence that HIV monoinfection increases the risk for HCC, our finding that HIV infection was associated with HCC—even after adjusting for HCV and HBV infection—should be interpreted with caution. One possible explanation is that HIV infection in our study population was associated with another major risk factor for HCC, such as alcohol abuse.
A comparison of the present study with a previous investigation of the Texas prison population reveals that a number of characteristics associated with an increased risk of HCC (e.g., male gender, Hispanic ethnicity, advanced age, HIV infection, and HCV infection) were also associated with end-stage liver disease. The concordance of these results is not surprising given the similar biological pathways of these two diseases. In fact, within our study population, 44% of inmates diagnosed with HCC were also diagnosed with end-stage liver disease.
To our knowledge, this study is the first epidemiologic investigation of HCC prevalence and mortality in a prison population. One of the strengths of our study was the large size of the study cohort (325,477 inmates). Because this investigation was carried out in the nation's largest state prison system, these findings have a high degree of statistical power and are likely to be generalizable to other prison systems in the U.S. An additional strength was that multiple sources were used to identify inmates with a diagnosis of HCC and those who died from HCC. Data sources included the TDCJ EMR system, TDCJ mortality record system, and UTMB cancer registry.
Limitations
The study also had several limitations. First, the TDCJ does not have a universal HCV or HBV screening policy. Although a previous blinded seroprevalence study of nearly 4,000 TDCJ inmates showed an overall HCV prevalence of 29%,6 the prevalence of HCV in the present study cohort was only 13%. These discrepant results suggest that more than 50% of TDCJ inmates with HCV may not have been identified in the present study. In contrast, the prevalence of HBV in the present study (approximately 2%) is comparable to earlier seroprevalence estimates for the TDCJ population (Unpublished data, John Pulvino, PA, TDCJ, 2001). Because the presence of chronic HBV infection in HIV-negative inmates may be more likely to result in clinical symptoms than chronic HCV infection, it is possible that HBV infection was more likely to have been diagnosed than HCV infection in our study population.
Second, because information on the overall number of inmates who were screened for HIV, HCV, and HBV was available only in aggregate form, we were not able to include this information in our analyses. Consequently, inmates who were infected with HIV, HCV, or HBV but who were not tested may have been misclassified as uninfected. It is difficult to determine the extent to which such misclassification affected our assessment of the exposure-disease association. If inmates who were positive but untested had an increased risk of developing HCC—perhaps mediated by risk behaviors such as long-term alcohol abuse—then the failure to include these inmates in their true risk-factor category (e.g., HCV-positive) would have produced results that were biased toward the null hypothesis (no association). Alternatively, if untested but infected inmates actually had a reduced risk of HCC prevalence or mortality, then failure to accurately measure their infection status would have resulted in an inflated risk factor-disease association. Consideration of such biases is particularly relevant in interpreting HCV-related findings, given our estimation that 50% of HCV-infected inmates were undiagnosed.
Third, as with any study conducted in a correctional setting, tracking inmates after release from prison is difficult. Consequently, we had to limit our analyses of HCC mortality to inmates who died during incarceration. Overall, less than 2% of inmates with HCC were granted early medical release during the study period. However, because the vast majority of inmates serve sentences for less than three years, the HCC mortality in this study population would likely be underestimated.
Finally, because complete screening information was not available for a substantial proportion of the study cohort, we were not able to ascertain the degree of adherence to standard screening for HCC in the prison population via serial alpha-fetoprotein and ultrasound, a practice that might reduce HCC mortality rates.
CONCLUSIONS
The exceedingly high prevalence of HCC and associated mortality identified in this study population suggests that correctional health-care providers and policy makers will face significant challenges over the next decade. Most individuals currently infected with HCV were born between 1945 and 1964, and were likely infected from the late 1960s through the 1980s.20 As a consequence of the increased duration of prison sentences in the U.S., a substantial number of offenders from this birth cohort will remain incarcerated for many years. Because the onset of cirrhosis typically does not occur for at least 20 to 30 years after initial HCV infection, it is anticipated that the number of new HCC cases will continue to rise over the next decade.19 In view of the high concentration of HCV infections among incarcerated populations, this epidemic will place an inordinate burden on prison systems. Consequently, it will be important for correctional systems—perhaps in partnership with one another—to validate the efficacy of screening programs for inmates at high risk of HCC and to implement comprehensive prison-based prevention and treatment programs for hepatitis.
Acknowledgments
The authors thank Owen Murray, DO, Fred Wacker, PhD, and John Pulvino, PA, for critically reviewing the article and Leonard Pechacek for editorial and writing assistance.
REFERENCES
- 1.El-Serag HB, Davila JA, Peterson NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139:817–23. doi: 10.7326/0003-4819-139-10-200311180-00009. published erratum appears in Ann Intern Med 2004;140:151. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–50. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 3.McGlynn KA, Tarone RE, El-Serag HB. A comparison of trends in the incidence of hepatocellular carcinoma and intrahepatic cholangiocarcinoma in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15:1198–203. doi: 10.1158/1055-9965.EPI-05-0811. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB, Mason AC. Risk factors for the rising rates of primary liver cancer in the United States. Arch Intern Med. 2000;160:3227–30. doi: 10.1001/archinte.160.21.3227. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong GL, Alter MJ, McQuillan GM, Margolis HS. The past incidence of hepatitis C virus infection: implications for the future burden of chronic liver disease in the United States. Hepatology. 2000;31:777–82. doi: 10.1002/hep.510310332. [DOI] [PubMed] [Google Scholar]
- 6.Baillargeon J, Wu H, Kelley MJ, Grady J, Linthicum L, Dunn K. Hepatitis C seroprevalence among newly incarcerated inmates in the Texas correctional system. Public Health. 2003;117:43–8. doi: 10.1016/s0033-3506(02)00009-4. [DOI] [PubMed] [Google Scholar]
- 7.Macalino GE, Vlahov D, Sanford-Colby S, Patel S, Sabin K, Salas C, et al. Prevalence and incidence of HIV, hepatitis B virus, and hepatitis C virus infections among males in Rhode Island prisons. Am J Public Health. 2004;94:1218–23. doi: 10.2105/ajph.94.7.1218. published erratum appears in Am J Public Health 2004;94:1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinbaum CM, Sabin KM, Santibanez SS. Hepatitis B, hepatitis C, and HIV in correctional populations: a review of epidemiology and prevention. AIDS. 2005;19(Suppl 3):S41–6. doi: 10.1097/01.aids.0000192069.95819.aa. [DOI] [PubMed] [Google Scholar]
- 9.Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–62. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 10.Spaulding A, Stephenson B, Macalino G, Ruby W, Clarke JG, Flanigan TP. Human immunodeficiency virus in correctional facilities: a review. Clin Infect Dis. 2002;35:305–12. doi: 10.1086/341418. [DOI] [PubMed] [Google Scholar]
- 11.Baillargeon J, Soloway RD, Paar D, Giordano TP, Murray O, Grady J, et al. End-stage liver disease in a state prison population. Ann Epidemiol. 2007;17:808–13. doi: 10.1016/j.annepidem.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Fazel S, Bains P, Doll H. Substance abuse and dependence in prisoners: a systematic review. Addiction. 2006;101:181–91. doi: 10.1111/j.1360-0443.2006.01316.x. [DOI] [PubMed] [Google Scholar]
- 13.Peters RH, Greenbaum PE, Edens JF, Carter CR, Ortiz MM. Prevalence of DSM-IV substance abuse and dependence disorders among prison inmates. Am J Drug Alcohol Abuse. 1998;24:573–87. doi: 10.3109/00952999809019608. [DOI] [PubMed] [Google Scholar]
- 14.SAS Institute, Inc. SAS: Version 8.0. Cary (NC): SAS Institute, Inc.; 1999. [Google Scholar]
- 15.Ries LAG, Melbert D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, et al., editors. Surveillance epidemiology and end results (SEER) cancer statistics review, 1975–2004. Bethesda (MD): National Cancer Institute; 2007. [cited 2007 Nov 1]. Also available from: URL: http://seer.cancer.gov/csr/1975_2004. based on November 2006 SEER data submission, posted to the SEER website, 2007. [Google Scholar]
- 16.Seeff LB, Hoofnagle JH. Epidemiology of hepatocellular carcinoma in areas of low hepatitis B and hepatitis C endemicity. Oncogene. 2006;25:3771–7. doi: 10.1038/sj.onc.1209560. [DOI] [PubMed] [Google Scholar]
- 17.Braithwaite RL, Hammett TM, Mayberry RM. Prisons and AIDS: a public health challenge. San Francisco: Jossey-Bass Publishers; 1996. [Google Scholar]
- 18.El-Serag HB. Epidemiology of hepatocellular carcinoma in USA. Hepatol Res. 2007;37(Suppl 2):S88–94. doi: 10.1111/j.1872-034X.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- 19.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127(5 Suppl 1):S27–34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–14. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 21.Hassan MM, Hwang LY, Hatten CJ, Swaim M, Li D, Abbruzzese JL, et al. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36:1206–13. doi: 10.1053/jhep.2002.36780. [DOI] [PubMed] [Google Scholar]
- 22.Cowie CC, Rust KF, Byrd-Holt DD, Eberhardt MS, Flegal KM, Engelgau MM, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health and Nutrition Examination Survey 1999–2002. Diabetes Care. 2006;29:1263–8. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- 23.Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care. 1998;21:518–24. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 24.Degos F, Tural C. Hepatocellular carcinoma in human immunodeficiency virus (HIV)-infected patients: is it really different, and if so, why? J Hepatol. 2007;47:447–50. doi: 10.1016/j.jhep.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Bica I, McGovern B, Dhar R, Stone D, McGowan K, Scheib R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32:492–7. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 26.Soto B, Sanchez-Quijano A, Rodrigo L, del Olmo JA, García-Bengoechea M, Hernández-Quero J, et al. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol. 1997;26:1–5. doi: 10.1016/s0168-8278(97)80001-3. [DOI] [PubMed] [Google Scholar]
- 27.Benhamou Y, Bochet M, Di Martino V, Charlotte F, Azria F, Coutellier A, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30:1054–8. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 28.Telfer PT, Brown D, Devereaux H, Lee CA, DuSheiko GM. HCV RNA levels and HIV infection: evidence for a viral interaction in haemophilic patients. Br J Haematol. 1994;88:397–9. doi: 10.1111/j.1365-2141.1994.tb05038.x. [DOI] [PubMed] [Google Scholar]
- 29.Martin P, Di Bisceglie AM, Kassianides C, Lisker-Melman M, Hoofnagle JH. Rapidly progressive non-A, non-B hepatitis in patients with human immunodeficiency virus infection. Gastroenterology. 1989;97:1559–61. doi: 10.1016/0016-5085(89)90405-8. [DOI] [PubMed] [Google Scholar]