Figure 2.
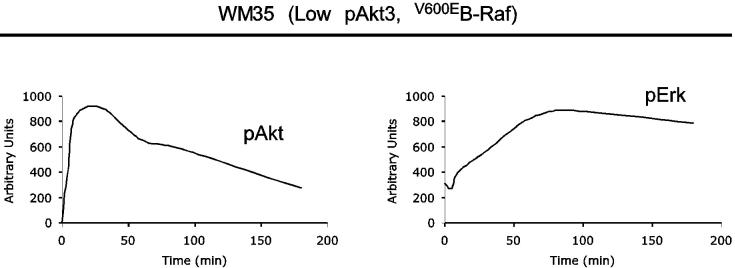
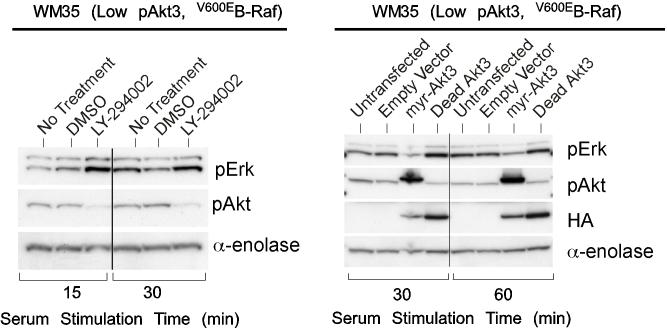
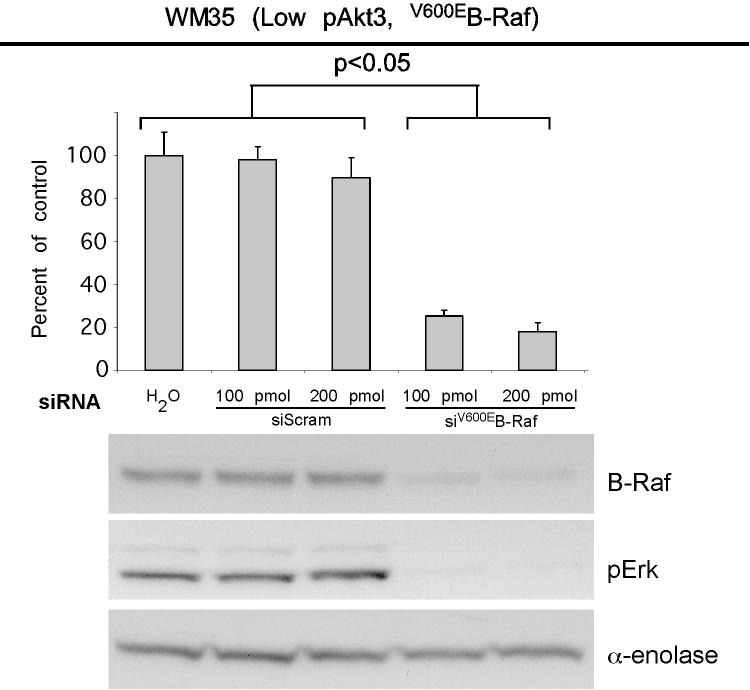
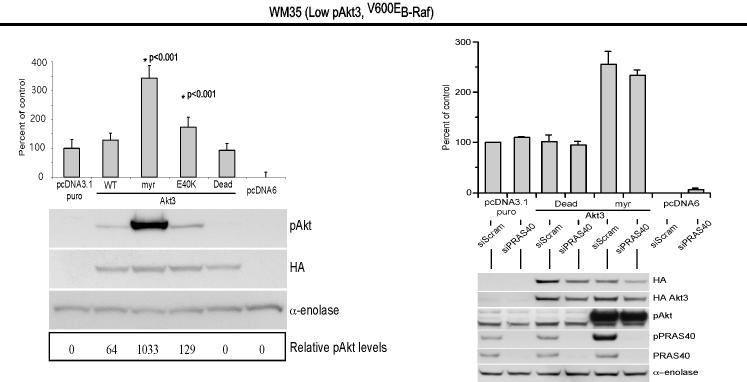
V600EB-Raf and Akt3 regulate anchorage independent growth of early melanoma cells. A, WM35 has constitutive activation of MAP kinase pathway signaling due to presence of V600EB-Raf but normal Akt activity. Densitometric quantitation of pAkt and pErk levels from Western blots of the WM35 cell line following 24 hours serum starvation followed by stimulation, shows constitutively high levels of pErk despite serum starvation, which is caused by V600EB-Raf activating the MAP kinase signaling cascade. In contrast, the Akt activation profile is similar to that observed for melanocytes. B, Inhibition of pAkt in WM35 cells using LY-294002 resulted in higher pErk while expression of active Akt3 decreased pErk levels. Treatment with LY-294002 led to a reduction in pAkt and a corresponding increase in pErk levels. Ectopic expression of active Akt3 decreased pErk levels. WM35 cells were nucleofected with Akt3 expressing constructs and following two days recovery, cells were starved 24 hours, and then stimulated with serum containing media and protein lysates collected for Western blot analysis. Ectopic myristoylated-Akt3 expression led to decreased pErk levels. C, Inhibition of B-Raf decreases anchorage independent growth of early melanoma cells. WM35 cells were nucleofected with siRNA targeting V600EB-Raf (100 and 200 pmoles), scrambled siRNA, or a water control. Cells were then plated onto PolyHEMA coated 96 well plates to measure effect on anchorage independent growth. MTS assay, 3 days later, was used to quantify viable cell number. Western blot showed efficient knockdown of B-Raf protein expression and decreased pErk levels. α-enolase served as a control for protein loading. D, Ectopic expression of active Akt3 enhanced anchorage independent growth. Viability of WM35 cells nucleofected with Akt3 is shown along with Western blot analysis of cell lysates. WM35 cells were nucleofected with various Akt3 expressing constructs: empty vector, WT-Akt3, Dead-Akt3 and active Akt3: myristoylated-Akt3 (myr-Akt3) or E40K-Akt3 alone or in combination with a siRNA targeting PRAS40 protein. After nucleofection, cells were plated directly onto PolyHEMA coated 96 well plates and following 3 days of growth in selection media, an MTS assay was used to quantify cell viability. Myr-Akt3 significantly increased cell growth compared to less active E40K-Akt3 that had a more modest effect. Changes in cell growth corresponded directly to pAkt level, which is shown by Western blot analysis and quantified by densitometry. Knocking down PRAS40 had no significant effect on cell viability. HA probed Western blot shows equal ectopic expression of the different Akt3 proteins.