Figure 3.
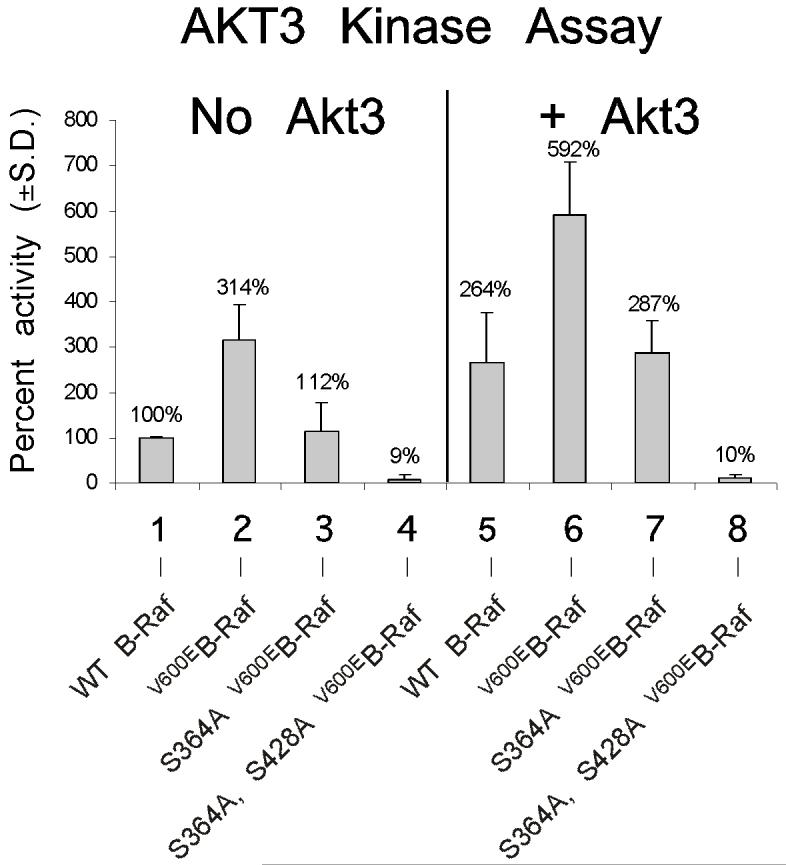
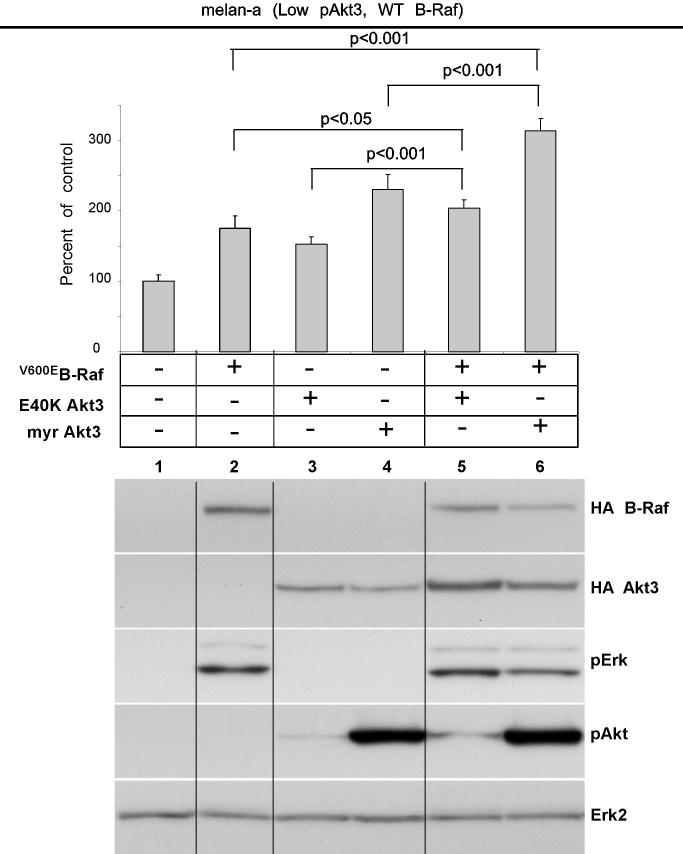
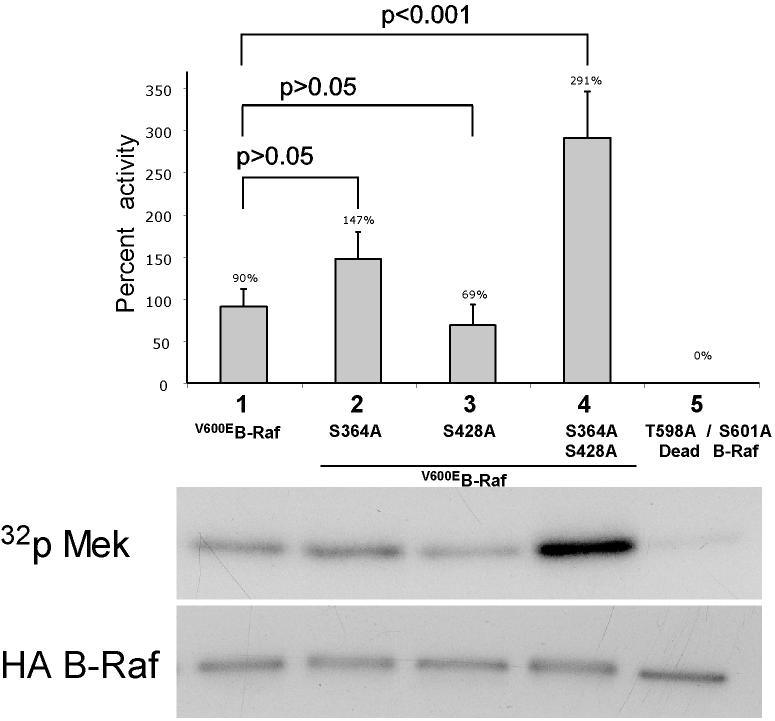
V600EB-Raf and Akt3 cooperate in melanocyte transformation. A, Melan-a transformation, following ectopic V600EB-Raf and Akt3 expression, was quantified by determined effect on anchorage independent growth. Melan-a cells were nucleofected with various combinations of V600EB-Raf and Akt3 expression plasmids (resistant to blasticidin and puromycin, respectively) and then directly plated onto PolyHEMA coated 96 well plates. Transfected cells were selected for blasticidin and puromycin resistance for three days followed by MTS assay to quantify cell viability. Results of a representative MTS assay are shown indicating effects of V600EB-Raf and Akt3 expression on anchorage independent growth. Nucleofections of either V600EB-Raf or myr-Akt3 led to increased cell viability; however, co-expression of both genes resulted in a more statistically significant effect. Western blots indicate changes in signaling pathways following ectopic expression of B-Raf and Akt3. Error bars; mean ± S.D. B, In vitro B-Raf kinase assay showing activity of V600EB-Raf compared to protein in which phosphorylation sites are converted to alanine S364A and S428A. Upper blot show autoradiography film of 32P labeled Mek substrate while lower shows a Western blot quantifying the amount of immunoprecipitated B-Raf protein used for the kinase assay. The graph represents average densitometry analysis (±S.D.) of three independent experiments, where values indicate level of labeled Mek normalized to the amount of HA B-Raf, relative to Dead B-Raf set as the background control. C, Akt3 phosphorylates V600EB-Raf on serines 364 and 428. Quantitation of B-Raf phosphorylation using densitometric scans of Western blot that were normalized to total immunoprecipitated HA B-Raf. Both wild type and V600EB-Raf were phosphorylated by Akt3. Conversion of serine 364 to alanine reduced levels of V600EB-Raf phosphorylation, while mutation of both serines 364 and 428 completely abrogated phosphorylation by Akt3. Graph is the average of three independent experiments; error bars, mean ±S.D.