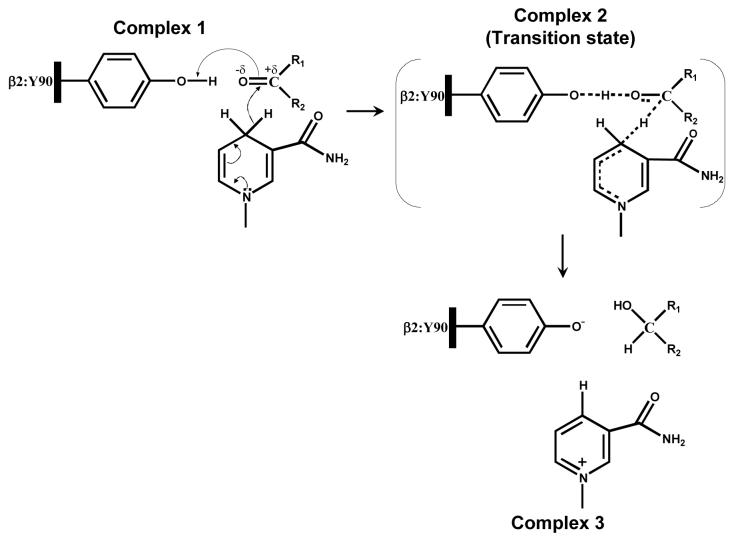
Scheme 3. Mechanism of NADPH-dependent carbonyl reduction catalyzed by Kvβ2.
In complex 1, Tyr-90 is shown poised to form a hydrogen bond with the substrate carbonyl which results in carbonyl polarization, accelerating the hydride transfer of the pro-R hydrogen from the nicotinamide ring of NADPH to the carbonyl carbon of the substrate. Complex 2 shows a non-charged transition state in which the polarization at the carbonyl is quenched by the proton transfer from the protein tyrosine and a concerted hydride transfer to the carbonyl carbon. The reduced carbonyl then dissociates from the acid-base catalyst and a net charge develops on the oxidized cofactor and the tyrosinate anion (Complex 3).