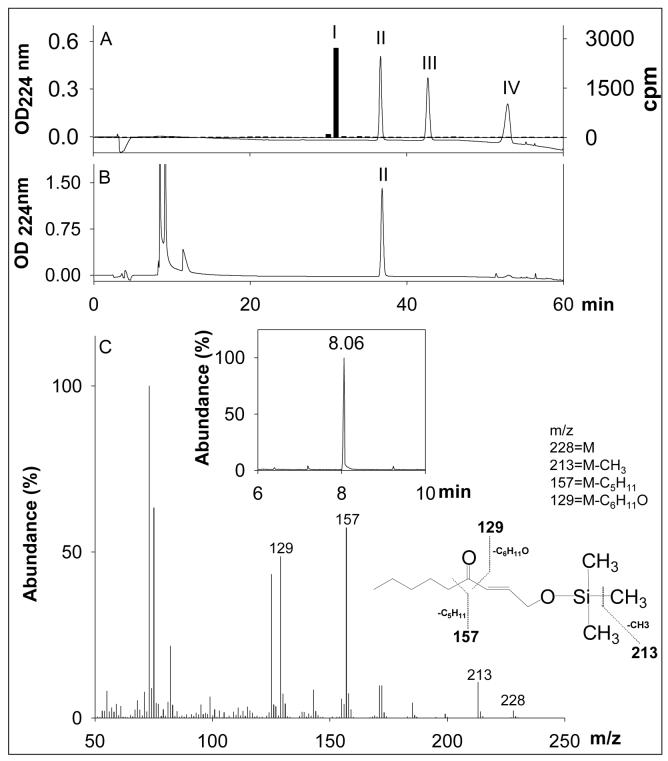
Figure 4. Identification of Kvβ2-derived product of 4-oxo-2-nonenal (4-ONE).
Panel A shows the separation of reagent 3H-1,4-dihydroxynonenol (DHN; 3000 cpm), 4-oxo-2-nonenol, 4-hydroxy-2-nonenal (4-HNE) and 4-ONE, 40 nmoles each on ODS C18 reverse phase HPLC column. Y-axis on the right side shows the radioactivity profile of 3H-DHN whereas Y-axis on the left side shows OD224. Peaks I-IV eluting with retention times of 31, 37, 43 and 53 min are due to reagent DHN, 4-oxo-2-nonenol, 4-HNE and 4-ONE, respectively. Panel B shows the HPLC traces of the Kvβ2-catalyzed reduction product of 4-ONE. Reagent 4-ONE (100 nmoles) was incubated with 0.6 mg Kvβ2 and 0.3 mM NADPH in 0.2 M potassium phosphate for 2h at 37 °C and the product was separated on HPLC. Panel C: inset shows a representative gas chromatogram of derivatized peak II (from panel B), eluting at 8.06 min and panel C shows the electron impact ionization-mass spectrum of the siliated metabolite of the GC peak eluting at 8.06 min. Ion with m/z 228 represents moleclular ion (M) of 4-oxo-2-nonenol. Ions with m/z 213, 157 and 129 respectively, correspond to M-CH3, M-C5H11 and M-C6H11O fragments of 4-oxo-2-nonenol.