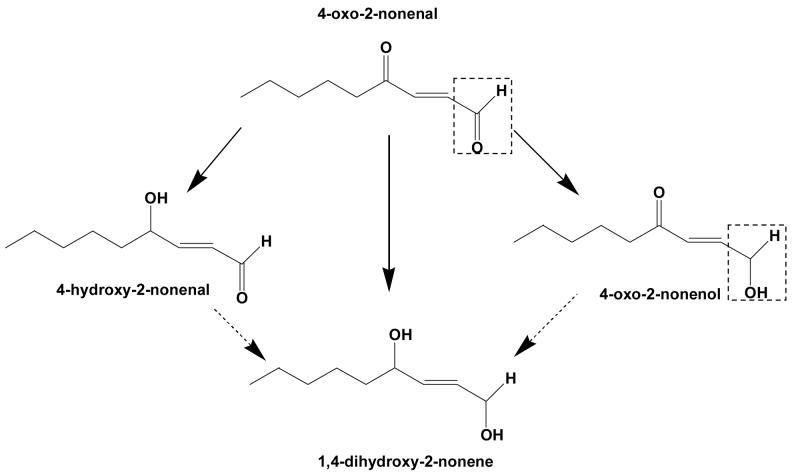
Scheme 1. Potential products of 4-oxo-2-nonenal (ONE) reduction.
Reduction of 4-oxo-2-nonenal at C-1 aldehyde would lead to the formation of 4-oxo-2-nonenol, whereas reduction of the C-4 keto group should yield 4-hydroxy-2-nonenal (HNE). Additional reduction of HNE or 4-oxo-2-nonenol would form 1,4-dihydroxy-2-nonenal (DHN). Note: Reduction in the presence of Kvβ2 led to the appearance of only 4-oxo-2-nonenol and no HNE or DHN formation was observed (see, Fig. 4). The reduction of the C-1 aldehyde to an alcohol is indicated by a dotted box.