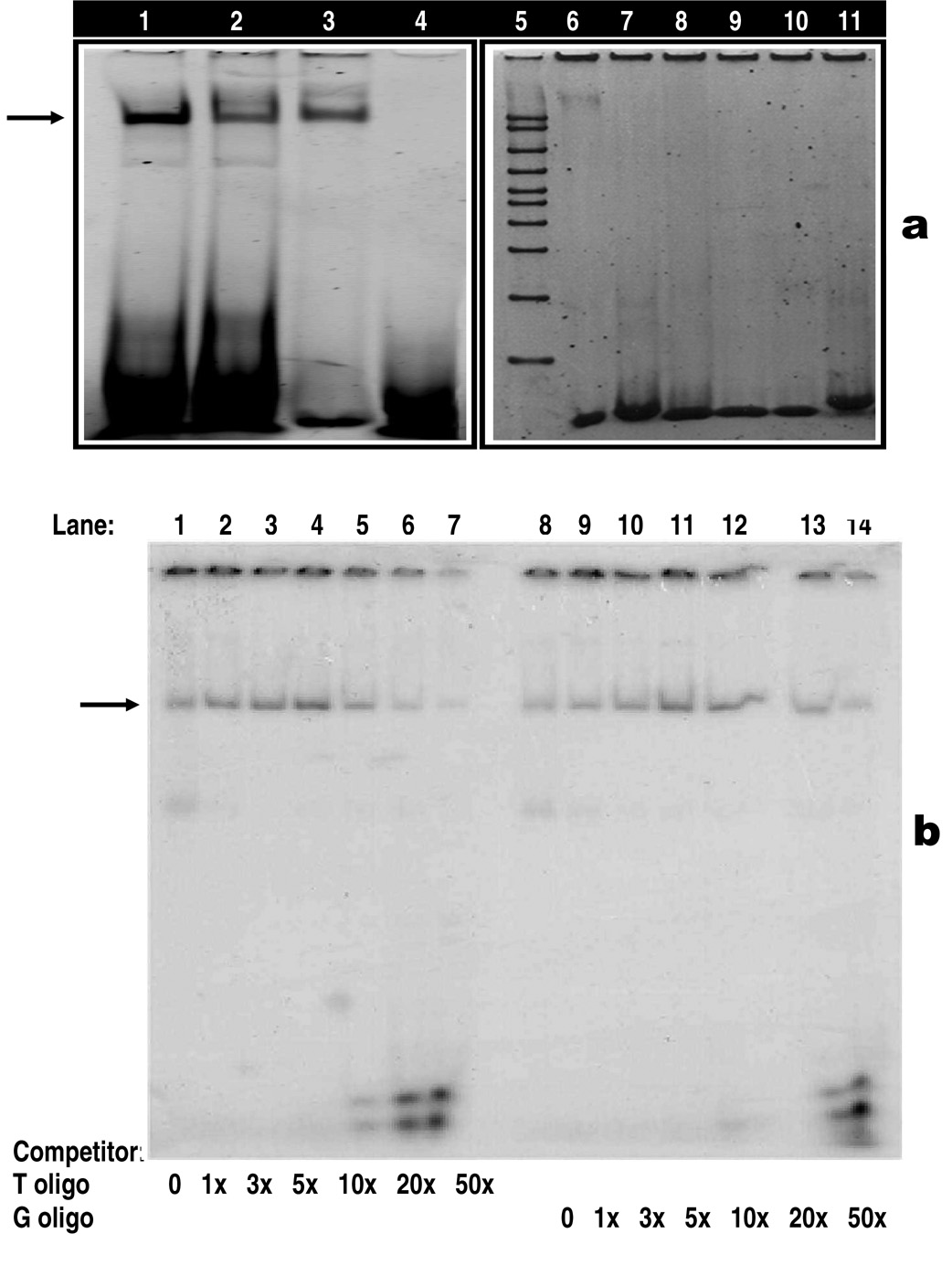
Figure 2.
a: Non-radioactive electrophoretic mobility shift assay (EMSA). Arrow head indicates the specific DNA-protein binding (lanes 1–3, 6) or no DNA-protein binding (lanes 7–11). Binding of HindIII wild type (lane 1) and mutant type (lane 2) with purified human TATA binding protein (TBP). Lanes 1 and 2 contain identical amounts of HindIII oligos and TBP, but the affinity of TBP is higher for the HindIII wild type (lane 1) than the mutant type (lane 2). Lanes 3 and 6 are positive controls showing the binding of TBP with TFIID consensus oligo. Purified TBP used in Lane 3 was obtained from Promega, whereas purified TBP used in lane 6 comes from Santa Cruz Biotechnologies and this may explain the difference in the intensity between the two bands. Lanes 4, 7–11 are negative controls showing no binding of TBP with consensus oligos, including AP2 (lanes 4 and 7), SP1 (lane 8), AP1 (lane 9), OCT1 (lane 10) and CREB (lane 11). Lane 5 contains 50 – 2000 bp marker.
b: Radioactive competitor EMSA for LPL/HindIII T→G polymorphism. Each sample contains a mixture of 5 µg of nuclear extract derived from aorta smooth muscle cells and a 30-mer 32P-labeled LPL/HindIII oligonucleotide containing the T allele. The arrowhead indicates the specific DNA-protein complex associated with the LPL/HindIII T→G polymorphic site. Competition assay performed by adding excess cold oligonucleotides containing either the T allele (lanes 2–7) or the G allele (lanes 9–14). Lanes 1 and 8: no competitor, lanes 2–7 have increasing amounts of T oligo competitor (1x, 3x, 5x, 10x, 20x and 50x, respectively); lanes 9–14 have increasing amounts of G oligo competitors (1x, 3x, 5x, 10x, 20x and 50x, respectively).