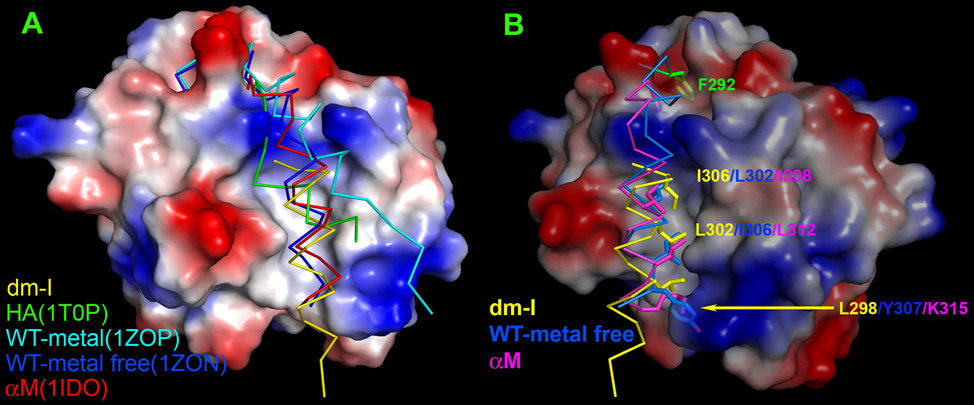
Figure 3. Diverse orientation of α7-helix.
I domains from different structures are superimposed. (A) Front view of a surface representation of the I domain from ICAM-5 complex with α7-helices from other structures overlaid. Note that the 5 α7-helices assume their distinct orientations within the conic groove, respectively. (B) Side view of Fig. 3A. Only α7-helices from I domain in ICAM-5 complex, αL I domain in metal-free form and αM I domain are shown for clarity. Hydrophobic residues forming hydrophobic interactions with the groove are shown with side chains. They are Leu302, Ile306 and Tyr307 in metal-free αL I domain as well as Ile308, Leu312 and the aliphatic portion of Lys315 in αM I domain from the top down. For the dm-I domain, they are Ile306, Leu302 and Leu298 in a reverse order. Also noticeable is the relatively hydrophobic pocket at the top of the figure, which accommodates the phenol ring of Phe292 (in green shade) in its closed form.