Figure 3. MLK3 depletion increases Rho GTPase and inhibits Rho-dependent SRF activation stimulated by Gαq.
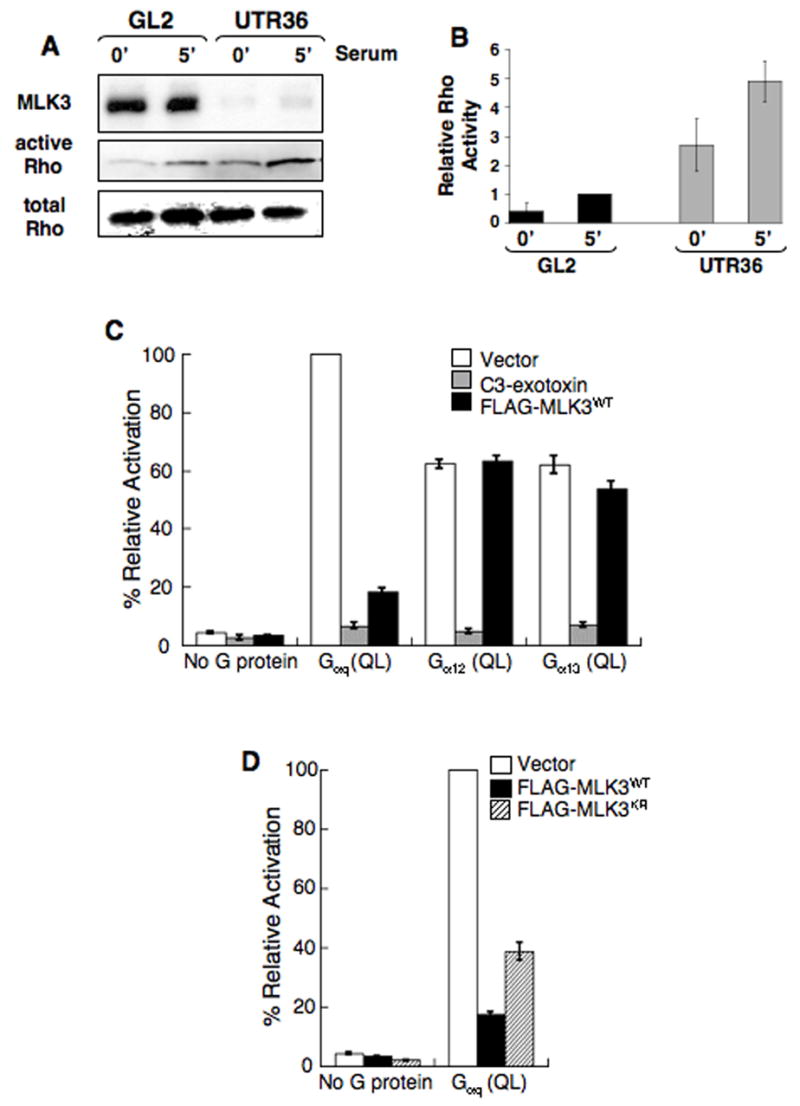
(A,B) Increased activated Rho is present in MLK3-depleted A549 cells. A549 cells transfected either with GL2 control siRNA or MLK3 UTR36 siRNA were serum-starved for 18 h then stimulated with serum for 5 min before preparating cell lysates. Activated Rho-GTP was isolated using a Rhotekin pull-down assay.
(A) Immuno-blot of samples from the pull-down assay was reacted with anti-RhoA. In parallel, immuno-blots of equal amounts of total lysate were reacted with anti-MLK3 and anti-RhoA. Data are representative of 4 experiments.
(B) Quantitation of Rho for each sample shown in panel A, + samples from 3 additional experiments (data not shown), was obtained using fluorochrome-labeled secondary antibodies and the Odyssey® infrared imaging system (LI-COR Biosciences) or densitometry and the active Rho/total Rho ratio determined (Relative Rho Activity). The ratio average from 4 experiments (+/− SEM) is plotted to compare activated Rho levels (active Rho/total Rho for the 5 min GL2 sample for each experiment is set arbitrarily at 1).
(C,D) MLK3 inhibits Rho-dependent SRF activation stimulated by Gαq.
(C) 293T cells were cotransfected with expression plasmids encoding either wild-type MLK3 (FLAG-MLK3WT), C3 exotoxin, or empty vector and constitutively active, GTPase-deficient Gαq (Q229L), Gα12 (Q229L), Gα13 (Q226L) or no G protein (empty vector alone). Transfections also included a firefly luciferase reporter plasmid that is responsive to SRF activation (pSRF-Luc) and a plasmid which expresses Renilla luciferase (phRG-TK) as an internal control. 19 h later, cell lysates were prepared and assayed for dual luciferase activities. Data represent firefly luciferase activity normalized to Renilla luciferase activity, expressed as % activation relative to that elicited by Gαq (QL) in cells transfected also with empty vector control. Total cell lysates were analyzed by immuno-blot with anti-FLAG,-Gαq,-Gα12 or -Gα13 (see Supplemental Figure 3). The data are the average of 3 experiments (+/− SEM).
(D) MLK3 inhibition of Gαq-stimulated SRF activation is not dependent on catalytic activity. 293T cells were co-transfected with expression plasmids encoding either wild-type MLK3 (FLAG-MLK3WT), an ATP-binding point mutant of MLK3 (FLAG-MLK3KR) or empty vector and GTPase-deficient Gαq (Q229L) or no G protein (empty vector alone). SRF reporter plasmids were included in these transfections, samples were analyzed for SRF activation and data expressed as described in (C). Total cell lysates were immuno-blotted with anti-FLAG or anti-Gαq (see Supplemental Figure 3). The data are the average of 3 experiments (+/− SEM).
