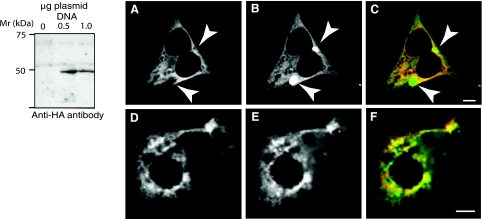
Fig. 11.
Western blot analysis of CAMK2δ6 and subcellular localization of CAMK2δ6 and D52 proteins in HEK293 and T84 cells. A: Western blotting demonstrated that the expressed protein product of TV3 (CAMK2δ6/G) has a migration pattern that is similar to D52 kinase and CAMK2α. cDNA encoding for HA-tagged CAMK2δ6/G was generated by PCR using the pGEM-T Easy-CAMK2δ-V3 construct (Fig. 10C) as a template then subcloned into pcDNA 3.1. PCR primers were as follows: forward with EcoRV cut site, 5′-CCC GAT ATC ATG GGC TAC CCA TAC GAT GTT CCA GAT TAC GCT GCT TCG ACC ACC ACC; reverse with XhoI cut site, 5′-CGC CTC GAG TTA GTT GAT GGG TAC TGT GGG. pcDNA 3.1 plasmids with and without the cDNA insert were transfected into HEK293 cells as described in methods. The expressed protein was identified by Western blotting using anti-HA antibody. B: CAMK2δ6 and D52 proteins display an overlapping subcellular distribution when coexpressed in HEK293 and T84 cells. Both cell lines were transfected with Effectene by using 0.5 μg plasmid DNA for each construct as described in methods. HA-tagged CAMK2δ6 was immunolocalized with monoclonal anti-HA antibody (1:1,000 dilution) in conjunction with donkey anti-mouse Cy-5-tagged secondary antibody (1:100 dilution) (A and D) and pseudocolored in red in the merged images (C and F). enhanced green fluorescent protein (GFP; EGFP)-tagged D52 was analyzed directly (B) or after staining with a polyclonal anti-GFP antibody and donkey anti-rabbit Alexa 555 secondary antibody (1:100 dilution) (E) and pseudocolored in green, rather than blue, for better visualization in merged images (C and F). The absence of signal crossover was confirmed in all analyses. Arrows in A–C indicate accumulation of EGFP-tagged D52 within subcellular compartments from which CAMK2δ6 is excluded. This accumulation may, at least partially, reflect the “trapping” of the overexpressed EGFP-tagged protein within the Golgi apparatus. Images were acquired with a Zeiss LSM 510 confocal microscope using a ×40, 1.3-NA oil immersion objective. Bars, 5 μm.