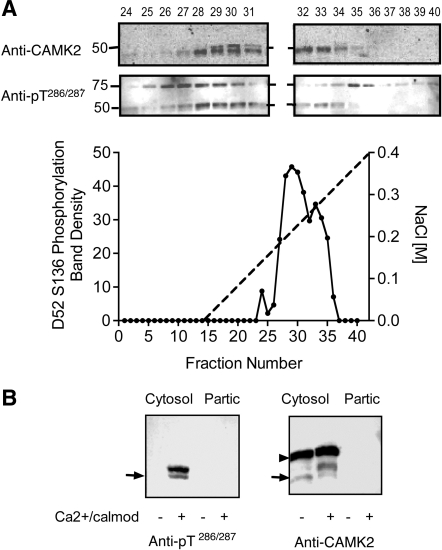
Fig. 8.
Characterization of D52 kinase in mouse gastric glands. A: D52 kinase activity profile in mouse gastric gland extracts after fractionation on a Mono Q column. Cells were extracted and D52 kinase activity was assayed as described in Fig. 7. Inset shows patterns of cross-reactivity of column fractions with anti-CAMK2 and anti-pT286/287 following stimulation with calcium/calmodulin. The identity of the ∼75-kDa protein(s) that cross-reacted with the anti-CAMK2 antibody is unknown. B: D52 kinase activity in mouse gastric gland extract before column fractionation. Glands were sonicated in lysis buffer and centrifuged (100,000 g, Cytosol) as described in methods. Pellets were extracted with 0.1% Triton X-100 (Partic) then assayed along with the cytosolic extract. Left: Western blot probed with anti-active CAMK2 (anti-pT286/287). Right: Western blot probed with anti-CAMK2. Arrow indicates position of 50 kDa Mr standard. Although no CAMK2 kinase activity was detected in the particulate fraction in this experiment, minor activity (15–20% of the total) was detected in others (not shown). Note the prominent Mr band shift in CAMK2 signal following activation with calcium/calmodulin and absence of detectable pT286/287 signal when these activators were omitted. This band shift was not detected in HEK293 cell extracts (Fig. 7). The identity of the ∼60-kDa protein that cross-reacted with anti-CAMK2 antibody (arrowhead) but not the anti-active CAMK2 antibody is unknown; however, since this protein did not cross-react with anti-active CAMK2 antibody, it was presumed not to be a CAMK2 isoform.