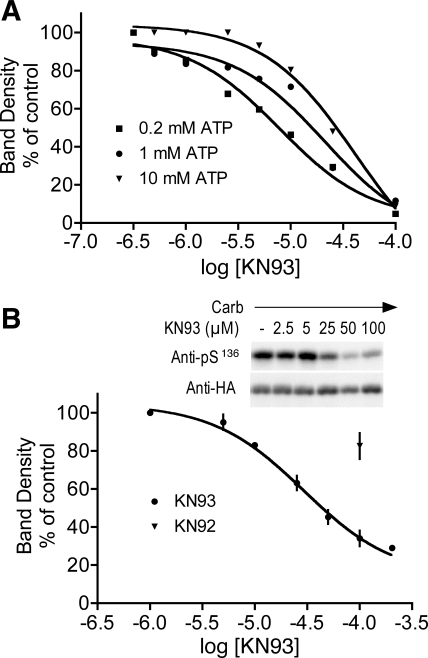
Fig. 9.
Comparison of effects of the CAMK2 inhibitor KN93 on S136 phosphorylation in intact cells and in vitro. A: in vitro analyses. D52 kinase was partially purified from HEK293 cells by fractionation on a Mono Q column. Peak activity fractions were identified, pooled as described in Fig. 7, and then assayed in the presence ATP (0.2, 1, and 10 mM) and varying concentration of KN93. The calmodulin concentration was kept constant at 200 nM. Changes in D52 S136 phosphorylation were quantified by Western blotting with affinity-purified pS136 antibody. Values are expressed as % of band densities obtained in the absence of KN93. For Ki calculations, the Kd for calmodulin binding was assumed to be 10 nM (18). In similar assays with fractionated mouse gastric gland extracts, the apparent IC50 with 0.2 mM ATP was 5.4 μM and the Ki was 0.26 μM (data not shown). B: cellular analyses. HEK293 cells expressing HA-tagged D52 were preincubated with varying concentrations of KN93 or with 100 μM KN92 or DMSO vehicle (where appropriate) for 1 h then stimulated with carbachol (10 μM, 1 min). Extracts were analyzed by Western blotting using affinity purified pS136 antibody. Corrections for variations in the levels of HA-D52 expression were performed as described in Fig. 5.