Abstract
Podocyte loss in adults leads to glomerulosclerosis. However, the impact of podocyte loss on glomerulogenesis and the development of the kidney as a whole has not been directly studied. Here, we used a podocyte-specific Cre transgene to direct the production of diphtheria toxin (DTA) inside podocytes during nephrogenesis. Affected podocytes underwent translational arrest and apoptosis, leading to oliguria, proteinuria, hematuria, interstitial hemorrhage, and perinatal death. Glomerular cell-cell interactions were disrupted, even before overt podocyte apoptosis. VEGF production by podocytes was greatly decreased, and this was associated with reduced endothelial fenestration and altered glomerular vascular architecture. In addition to these glomerular anomalies, embryonic podocyte ablation also led to structural changes and increased apoptosis in proximal tubules. The collecting ducts, however, only showed molecular changes that are likely an indirect effect of the greatly reduced urine flow. Although podocyte loss significantly impacted the development and maintenance of the vasculature both inside and outside the glomerulus, our results suggest that there is a lack of long-range signaling from deep-seated, mature glomeruli to the differentiating cells in the outer nephrogenic zone. This study illustrates the tight integration of various cell types in the developing kidney and shows that the impact of podocyte loss during development is much greater than that in adults. This study also shows the specificity and effectiveness of a genetically controlled podocyte ablation system in mice where the additional readily available tools can further expand its applications.
Keywords: kidney development, glomerulogenesis, diphtheria toxin
the glomerulus contains a number of unique cell types and structures to ensure the efficiency and specificity of blood filtration. The glomerular filtration barrier consists of the podocytes (glomerular visceral epithelial cells), fenestrated endothelium, and the intervening glomerular basement membrane (GBM) (19, 21). Glomerulogenesis is divided into the following stages: vesicle, comma- and S-shaped, glomerular capillary loop stage, and mature glomerulus. The renal vesicle consists of polarized cells surrounded by a basement membrane. The appearance of clefts in this structure produces the comma-shaped and then S-shaped figures. The lip beneath the distal cleft is established by a layer of epithelial cells that will differentiate into podocytes. As they enter the subsequent capillary loop stage, podocyte progenitors express podocyte markers and start to establish their foot processes as well as the slit diaphragm (21). The kidney becomes a functional excretory organ only after a sufficient number of glomeruli have developed along with their associated nephron tubules and collecting ducts. The epithelial cells in the glomerulus (podocytes and the parietal epithelium lining Bowman's capsule) and the epithelial cells of the renal tubules are derived from the metanephric mesenchyme by mesenchymal-epithelial transformation during nephrogenesis (19, 21). The collecting ducts, however, are derived exclusively from the ureteric bud. Nephrogenesis involves complex temporal and spatial integration of multiple cell types and can only be achieved through the precise orchestration of the proliferation, differentiation, and migration of various cell populations.
Podocyte loss has been found in many renal diseases and is associated with the development of proteinuria and glomerulosclerosis (23, 26). Surgical, chemical, and genetic ablation methods have been used to study the functional role of selected cell populations and to generate kidney disease models (8, 14, 17, 20, 25). Among these methods, genetically controlled cell ablation ensures the highest precision and consistency. In vivo cell ablation by diphtheria toxin (DTA) has proven to be an accurate and effective cell ablation method (13, 20, 25). DTA causes translational arrest and apoptosis through its inhibition of eukaryotic elongation factor 2 (6). Because rodents lack the receptor for DTA, DTA administration in rodents selectively eliminates cells with transgenic expression of the human DTA receptor (3, 22, 25). This strategy has been successfully used to study the pathological consequences of podocyte injury in adult rats (25). Alternatively, controlled endogenous expression of DTA in selected cells, as described in this paper, targets podocytes for destruction (1, 18). The use of an attenuated DTA, DTA176, prevents unintended cell death due to any minor leakiness of the promoters used (2, 27).
The high degree of functional interdependence among various renal cell populations from different embryonic origins predicts a tight coordination of communication and structural integration during nephrogenesis. Although previous experiments demonstrated an indispensable role for podocytes in glomerular function and renal disease progression in adults, the impact of embryonic podocyte disruption on the development of the glomerulus and the kidney as a whole has not been directly studied. Here we describe a novel mouse podocyte ablation model in which the Podocin promoter directs Cre-mediated DTA176 production and subsequent podocyte ablation during nephrogenesis. We have demonstrated the effectiveness and specificity of this system in mice, for which a large array of genetic tools is readily available for a broad range of analyses. Our study also demonstrates that podocytes have both important structural and signaling functions during development. Embryonic disruption of podocytes has profound impact not only in the glomerulus but also in other renal structures, leading to a much more devastating injury compared with podocyte loss in adults.
MATERIALS AND METHODS
Mouse (Mus musculus) strains and urinalysis.
All animal studies have been approved by Institutional Animal Care and Use Committee at Washington University School of Medicine. The ROSA26-DTA176 and the Podocin-Cre strains were described previously (16, 27). The PCR genotyping methods are as follows. For Podocin-Cre, we used primers FCCreF1 TCGATGCAACGAGTGATGAG and FCCreR1 TCCATGAGTGAACGAACCTG to amplify a 420-bp product. PCR conditions were 95°C, 4′, 35 cycles (94°C 35″; 61°C, 35″; 72°C, 35″). For ROSA26-DTA176, we used primers WS268 GTTATCAGTAAGGGAGCTGCAGTGG, WS270 AAGACCGCGAAGAGTTTGTCCTC, and WS271 GGCGGATCACAAGCAATAATAACC to amplify a wild-type band of 415 bp and a band of 302 bp corresponding to the ROSA26-DTA176 allele. PCR conditions are: 95°C, 2′, 30 cycles (94°C 30″; 59.5°C, 30″; 72°C, 30″), 72°C, 5′. Urine was extracted from the bladders by using syringes fitted with 30-gauge needles. Urine samples were subjected to electrophoresis on a 10% polyacrylamide gel. Proteins are visualized by Biosafe Coomassie stain (Bio-Rad 161-0786).
Histology and electron microscopy.
Embryos or tissues were fixed with 4% paraformaldehyde and embedded in paraffin. Sections of 5 μm were collected and stained with hematoxylin and eosin. 5-Bromo-4-chloro-3-indolyl-d-galactoside staining on cryostat sections was performed as described (4). For transmission electron microscopy, tissues were fixed, embedded in plastic, and sectioned as previously described (9). For morphometric analysis of electron microscopic micrographs, we used the ImageJ program to analyze a total of 24,851 nm of GBM in control samples and 52,889 nm of GBM in mutant samples. Data were organized and processed in Microsoft Excel.
Immunohistochemistry.
Immunostaining on paraffin sections was performed as previously described (15). Antibodies used were rabbit polyclonal anti-PODOCIN (1:500, Sigma), mouse monoclonal anti-DESMIN (1:50, Developmental Study Hybridoma Bank), rabbit polyclonal anti-WT1 (1:100, Santa Cruz Biotechnology), rabbit polyclonal anti-aquaporin-2 (AQP2; 1:500, Chemicon), rabbit polyclonal anti-ClCK (1:100, Alamone Labs), and rabbit anti-PAX2 antibody (1:100, Covance). Alexa Fluor 488- or 555-conjugated secondary antibodies (1:1,000, Molecular Probes) were used to detect the corresponding primary antibodies. Both Dolichos biflorus agglutinin (DBA) and Lotus tetragonolobus agglutinin (LTA) were purchased from Vector Laboratories (used at 1:200).
RNA in situ hybridization and apoptosis assay.
RNA in situ hybridization was performed by using a DAKO Tyramide amplification kit (Dako, Carpinteria, CA) as previously described (5, 10). The in situ probe for VEGF was synthesized from a plasmid kindly provided by Dr. Gerald R. Crabtree (Stanford University). Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) analysis was performed by using the ApopTag plus peroxidase in situ apoptosis detection kit (Roche, Nutley, NJ). For the quantitative evaluation of the percentage of podocytes undergoing apoptosis in the control and mutants, we have included the immunostaining of PODOCIN on the same sections where TUNEL was performed. We studied 193 podocytes in the controls and 259 podocytes in the mutants. Data were organized and processed in Microsoft Excel.
RESULTS
Podocyte ablation during nephrogenesis causes hemorrhage, proteinuria, and perinatal lethality.
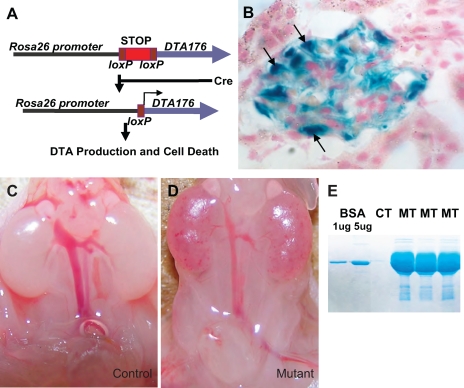
To study the impact of podocyte loss during nephrogenesis, we generated mice carrying both a Podocin-Cre (2.5P-Cre) transgene and a ROSA26-DTA176 allele. ROSA26-DTA176 has a loxP-flanked transcriptional stop cassette placed between the ubiquitous ROSA26 promoter and the coding sequence of DTA. This stop cassette prevents DTA transcription and cell death. However, in the presence of Cre, recombination between the loxP sites removes the stop cassette, leading to DTA transcription from the ROSA26 promoter and subsequent cell death (Fig. 1A). The Podocin-Cre transgene produces, through the Podocin promoter, podocyte-specific Cre expression as revealed by β-galactosidase assay on samples from mice carrying both Podocin-Cre and the ROSA26-LacZ reporter allele (Fig. 1B) (24). Every mouse carrying both Podocin-Cre and ROSA26-DTA176 (the “mutants”) died within 24 h of birth. while their littermates carrying one or none of these alleles (“controls”) were unaffected. The mutant kidneys displayed hyperemia, interstitial hemorrhage, and varying degrees of hypoplasia (Fig. 1, C and D). Additionally, mutant mice had significantly lower urine volumes (as determined by bladder content) and increased urine albumin (Fig. 1E). Evaluation of all other organs revealed no abnormalities.
Fig. 1.
Ablation of podocytes during nephrogenesis causes hemorrhage, proteinuria, and perinatal lethality. A: ROSA26-DTA176 allele has an attenuated diphtheria toxin (DTA; DTA176) knocked into the ROSA26 locus. A loxP-flanked transcriptional stop cassette prevents the production of DTA without induction. In the presence of Cre, loxP recombination removes the stop cassette, leading to DTA production, translational arrest, and apoptosis of the target cells. B: Podocin-Cre (2.5P-Cre) has specific expression in the podocytes as revealed by β-galactosidase assay on kidney section from a mouse carrying both the p2.5Cre transgene and the ROSA26R-lacZ reporter allele (24). Arrows are pointing at individual podocytes. C: kidneys of a newborn control mouse. D: kidneys of a newborn mutant mouse show interstitial hemorrhage and mild renal hypoplsia. E: mutants have heavy proteinuria at birth. CT, control; MT, mutant. Two microliters of urine was loaded onto each lane.
Cre-mediated DTA production induces podocyte apoptosis.
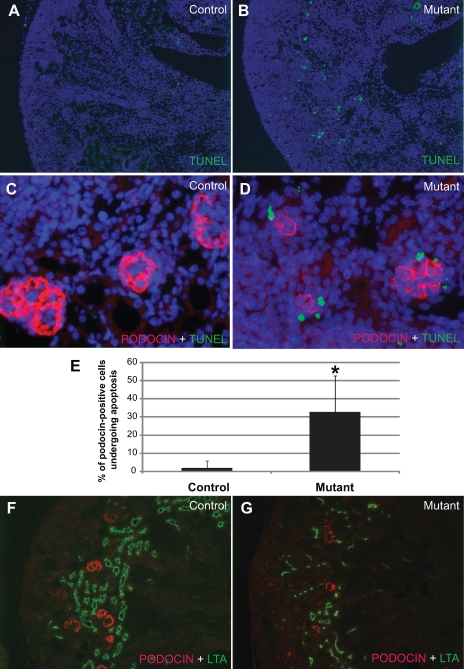
To reveal the cellular mechanisms leading to the observed abnormalities, we first performed TUNEL assays to examine potential changes in cell death associated with the Cre-induced DTA production. Dramatically increased numbers of apoptotic cells were noted in the mutant cortex (Fig. 2, A and B). Additional staining with an anti-PODOCIN antibody revealed that many podocytes were among the apoptotic cells in the mutant kidney (Fig. 2, C and D). In fact, while only 1.8 ± 4% of PODOCIN-positive cells (n = 193) were undergoing apoptosis in controls, ∼32.8 ± 20% of mutant podocytes (n = 259) were apoptotic (Fig. 2E). Additional apoptotic cells in the tubules are described below. The expression of PODOCIN should coincide with Cre expression from Podocin-Cre that initiates the sequence of events leading to apoptosis. Therefore, the PODOCIN-positive podocytes in the mutants are in the process of self-destruction. Since DTA causes translational arrest before overt apoptosis, it is possible that some podocytes lose PODOCIN expression first but persist and appear as PODOCIN negative (by immunostaining) before their eventual demise. Some of the mutant podocytes may have already been lost and were not included in the count in Fig. 2E. Thus this may represent an underestimation of the degree of podocyte apoptosis in the mutants. As a result of translational arrest and apoptosis, there are fewer PODOCIN-positive cells overall in the mutants (Fig. 2, F and G).
Fig. 2.
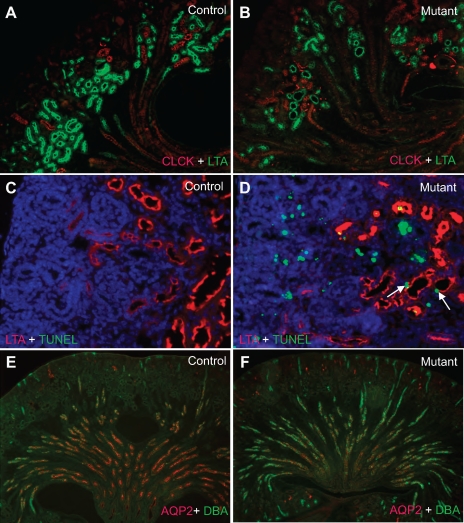
Cre-mediated DTA production induces podocyte apoptosis. All samples were taken from newborn mice. A and B: terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) staining of the kidney sections from a newborn control mouse (A) and a newborn mutant mouse (B). An increase in the number of apoptotic cells (green) is apparent in the mutant renal cortex. C and D: double staining with PODOCIN (red) and TUNEL (green) shows that PODOCIN-positive cells are present in the mutants (D), but with a dramatically higher level of apoptosis compared with the control (C). E: a significant difference in the percentage of podocytes undergoing apoptosis in the controls and the mutants is shown. As a result of podocyte apoptosis, there were overall a fewer number of PODOCIN-positive cells in the mutants (G) compared with the control (F). Lotus tetragonolobus agglutinin (LTA) labels the proximal tubules (green). *Significant difference (P < 0.0001).
DTA-mediated podocyte disruption during nephrogenesis leads to altered glomerulogenesis, eventual glomerular destruction, and interstitial hemorrhage.
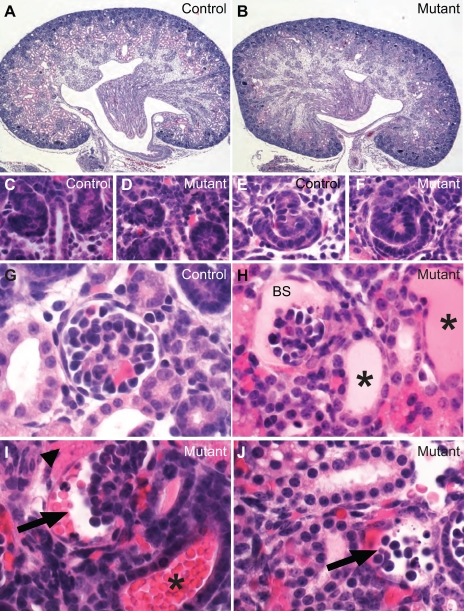
Although nephrogenesis continues until at least postnatal day 10 in mice, all cell types and structures in adult kidneys are found in newborn kidneys. Histopathological analyses revealed a range of developmental abnormalities in the mutant kidney within and outside the glomerulus (Fig. 3). Glomeruli at different developmental stages were found in the controls (Fig. 3, A, C, E, and G). The nephrogenic zone appeared relatively normal in the mutants, with developing glomeruli at the vesicle, comma-, and S-shaped stages (Fig. 3, B, D, and F). These early glomerular structures were similar between the controls and the mutants in morphology and in numbers (Fig. 3, A–F, and data not shown). Closer examination revealed that the more mature glomeruli in control mice had patent capillary loops, podocytes, endothelial cells, and mesangial cells (Fig. 3G). In the mutants, however, the glomeruli at similar positions appeared to be smaller and less developed (Fig. 3, H–J). Proteinaceous material was observed in Bowman's space in some mutant glomeruli and associated tubular lumen (Fig. 3H). In addition, there was apparent leakage of red blood cells (RBCs) into Bowman's space and the associated tubular lumens (Fig. 3I), suggesting compromised integrity of the filtration barrier. Glomeruli at various stages of collapse were also observed in the mutants (Fig. 3J). These collapsing glomeruli were in the process of losing cells and the overall glomerular architecture. Interstitial hemorrhage was frequently observed in areas surrounding the collapsing/collapsed glomeruli, likely as a result of further breakdown of Bowman's capsule by which the RBCs were first contained.
Fig. 3.
Podocyte loss during nephrogenesis causes extensive developmental and pathological changes in the glomeruli. A, C, E, and G: newborn control mice. B, D, F, and H–J: newborn mutants. These are all hematoxylin- and eosin-stained paraffin sections. The overall structural division is preserved in the mutant kidney with nephrogenic zone, cortex, and outer and inner medulla (B), although a number of anomalies were noted and will be described in this and other figures in more detail. Renal vesicles and S-shaped bodies are formed normally in the mutants (C and F). However, the mutant glomeruli have a variety of severe problems. Proteinaceous material was present in Bowman's space (BS) of some glomeruli and tubular lumens (* in H). Other glomeruli have red blood cells in Bowman's space (arrow in I) and the tubular lumen (* in I). Arrow in J points to a disintegrating glomerulus with apparent apoptotic bodies. Arrowhead (I) points to apparent extravasation of red blood cells in the interstitium adjacent to the glomerulus.
Malformation of the filtration barrier before overt podocyte apoptosis.
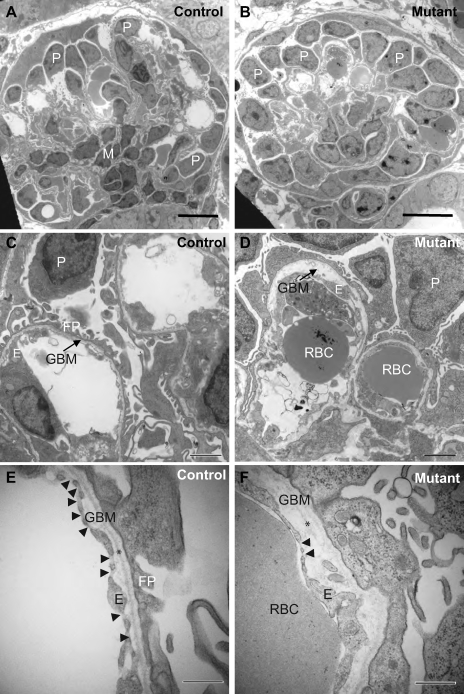
Electron microscopy was employed to examine ultrastructural changes within the developing glomeruli beyond the S-shaped body stage when the podocytes start to be affected by DTA but before massive apoptosis occurs. The mutant glomeruli analyzed were among the morphologically most advanced that we can find. In the control glomeruli at similar locations, podocytes were mostly seen in the periphery while mesangial cells were positioned closer to the center of the glomerulus supporting the capillary loops (Fig. 4A). While the mutant glomerulus had all the major cell types, the capillary loops appeared to be less divided (Fig. 4B). Defects in the mutant glomeruli were much better visualized in the higher magnification pictures. While the control GBM had well-structured podocyte foot processes on one side and the fenestrated endothelium on the other, mutant podocytes lacked well-defined foot processes, and the level of endothelial fenestration was reduced (Fig. 4, C–F). On average, the glomerular endothelium has 2.95 ± 1.16 fenestration/μm in the controls and 0.18 ± 0.19 fenestration/μm in the mutants (P < 0.001). Immunostaining revealed that the mutant GBM had the normal composition of laminins and collagen IV chains (data not shown). However, GBM thickness was extremely uneven in mutants. The lamina densa was discontinuous and even absent in many areas. There was apparent focal GBM splitting and greatly increased distance between podocytes and the endothelium (Fig. 4, C–F). It appears that major anomalies in glomerulogenesis have already occurred before overt apoptosis of the podocytes, likely due to the translational arrest and the loss of the signaling function of the podocytes.
Fig. 4.
Disruption of the filtration barrier before podocyte apoptosis. A, C, and E: electron microscopic micrographs of control glomeruli at P1. B, D, and F: electron microscopic micrographs of mutant glomeruli in similar positions at P1. Well-defined podocyte foot processes were present in the control glomeruli but essentially absent in the mutant glomeruli (C–F). The level of endothelial fenestration was reduced (C–F). Arrowheads in E and F point to endothelial fenestration. Glomerular basement membrane (GBM) thickness varied greatly in mutants. The distance between podocytes and the endothelium was greatly increased (D and F), likely due to the apparent focal GBM splitting (arrow in D). The lamina densa was visible in the control (* in E) but was discontinuous and even absent in many areas (* in F). Scale bars = 10 μm (A and B), 2 μm (C and D), 500 nm (E and F). P, podocyte; E, endothelial cell; M, mesangial cell; RBC, red blood cell; FP, foot processes.
Structural and molecular changes in tubules as a result of podocyte disruption.
In addition to glomerular defects, multiple abnormalities were observed in various segments of the nephron (Fig. 5). LTA staining revealed a minor reduction of proximal tubules in mutant kidneys (Figs. 5, A–D, and 2G). In addition, the structure of the proximal tubule lumen was highly variable. Most lumens were very small, but some were significantly dilated (Figs. 5, A–D, and 2G). A higher number of apoptotic cells in the proximal tubule epithelium and the lumen were seen compared with control (Fig. 5, C and D). The apoptotic cells in the proximal tubule lumen were presumably detached proximal tubule epithelium or apoptotic cells from the glomerulus. Distal tubules labeled by ClCK had narrower lumens than normal, although the changes were less prominent than in the proximal tubules (Fig. 5, A and B). While luminal narrowing may be explained by the greatly reduced urine output, occasional dilatation of the proximal tubules may be related to luminal obstruction by apoptotic debris. The basic structure of the collecting system was largely intact in mutants, but further analysis revealed a dramatic decrease in AQP2 expression (Fig. 5, E and F) that is most likely due to severe reduction of urine flow (12).
Fig. 5.
Nephron tubules developed in the presence of podocyte loss with structural alterations and molecular changes. A, C, and E: newborn controls. B, D, and F: newborn mutants. LTA (green) labels the proximal tubules. ClCK (red) labels distal tubules. Dolichos biflorus agglutinin (DBA; green) labels the collecting ducts. Increased apoptosis rate is also observed in the mutant proximal tubule epithelium (C and D, arrows). Most of the other TUNEL-positive cells are podocytes. Morphologically, the collecting ducts (labeled by DBA) seem to be largely unaffected by podocyte ablation. However, expression of aquaporin-2 (AQP2; red) in the collecting duct principal cells is significantly reduced in the mutant samples (F). Arrows point to the apoptotic cells in the proximal tubule epithelium.
Podocyte disruption alters VEGF signaling during nephrogenesis.
Nephrogenesis requires signaling interchanges among multiple cell types (21). To study the impact of embryonic podocyte disruption on the development of the kidney, expression of key developmental and cell type-specific markers and known signaling molecules was examined. Early nephrogenesis appeared normal in mutants, as PAX2-positive cells were organized into renal vesicles and other early nephron structures (Fig. 6, A and B). High-magnification images revealed WT1 expression in the cells of the lip beneath the cleft in the S-shaped body. These are podocyte progenitors that are not yet expressing PODOCIN and thus are not yet expressing DTA (Fig. 6, C and D, arrows). Mutants showed normal endothelial invasion of the cleft at this stage (Figs. 3, E and F, and 6, C and D). In addition, the proliferation rates of PAX2- and WT1-positive cells were similar in controls and mutants (data not shown). Although the possibility that developmental defects in deeper (and more mature) renal structures affect the emerging nephrons in the nephrogenic zone cannot be easily ruled out, at least in this developmental podocyte ablation model, such long distance effects are not evident. As the WT1-positive cells began to express PODOCIN and presumably DTA, they should become increasingly affected by the toxin. PECAM-positive endothelial cells and desmin-positive mesangial cells were present in mutant glomeruli, but the overall organization lacked the architectural complexity observed in control glomeruli at similar positions (Fig. 6, E–H).
Fig. 6.
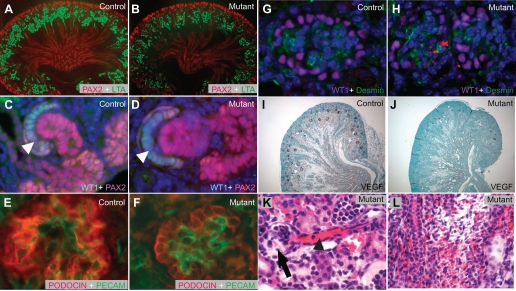
Podocyte ablation alters the interactions among major glomerular cell types. A and B: PAX2-positive cells (red) in the condensing mesenchyme within the nephrogenic zone are similar in the control and mutant samples in their numbers and distribution. C and D: WT1 (aqua) and PAX2 (red) staining revealed the podocyte progenitors (white arrowheads) and the tubular progenitors at S-shaped stage showing normal morphology and expression of the key regulatory genes. E and F: PECAM-1 and PODOCIN staining of control (E) and mutant (F) glomeruli at similar positions revealed abnormal glomerulogenesis in the mutants. G and H: disorganization of the glomeruli in the mutants is also revealed by WT1 (podocyte) and desmin (mesangial cell) labeling. Nevertheless, mesangial cells are present in the mutant glomeruli. I and J: RNA in situ hybridization on control (I) and mutant (J) sections showed that the normally high level of VEGF expression in the podocytes was greatly reduced in the mutants. K: dilated glomerular arterioles (black arrowhead) were observed in disintegrating glomeruli of mutants (arrow). L: interstitial hemorrhage in the medulla far away from the glomeruli in the mutants. All samples were taken from newborn mice.
As opposed to sudden death, the translational arrest before apoptosis in podocytes likely resulted in the loss of their function as a signaling source for glomerulogenesis well before their structural disintegration. Previous reports have indicated that VEGF secreted from the podocytes is essential for the development and maintenance of the glomerulus (7). We set out to investigate potential changes in VEGF production in the mutant kidney. RNA in situ hybridization on control mouse kidney sections revealed that the podocytes have a remarkably high level of VEGF expression (Fig. 6I). As a result of translational arrest and podocyte apoptosis, there was a dramatic decrease in VEGF transcripts in the mutant kidneys (Fig. 6J). The reduction in VEGF signaling certainly affects the ability of podocytes to communicate with endothelial cells (7), contributing to glomerular vascular abnormalities during glomerulogenesis before the disintegration of the glomerulus (Fig. 4). It is also possible that this reduction of VEGF signal has effects on the renal vasculature beyond the glomerular capillary loops. For example, dilatation of the glomerular arterioles (Fig. 6K) and interstitial hemorrhage in the medulla far away from the collapsed glomeruli (Fig. 6L) were seen in the mutants. Although these may be caused by hemodynamic changes as a result of the glomerular anomalies, the possibility exists that aberrant VEGF signaling during development may contribute to these changes.
DISCUSSION
Development and validation of a highly specific genetically-controlled podocyte ablation system in mice.
This study describes a podocyte ablation model based on genetically controlled and podocyte-specific internal production of diphtheria toxin in mice. The high specificity of this system results from the high specificity of the Podocin promoter and the use of the attenuated diphtheria toxin (DTA176) that prevents background leakage. Absence of the DT receptor in rodent cells also prevents any unintended damage to neighboring cells by DTA released from target cells undergoing apoptosis. The validation of the effectiveness and specificity of the Podcin-Cre/ROSA26-DTA176 murine model also sets the stage for genetically controlled conditional ablation of podocytes in mice based on internal DTA production. In fact, we are developing a murine doxycyclin-inducible system with sensitive control of the timing and extent of podocyte damage. Such a system will be useful not only for the study of the pathogenesis of glomerular disease but also for the creation of acute and chronic proteinuric models with a defined primary injury for treatment studies. This is the first description of the application in the kidney of a cell-ablation system based on internal production of DTA. Combined with other cell type-specific Cre transgenes, this system will be a valuable tool for investigating functional roles of specific cell populations and in generating disease models.
A different strategy for DTA-mediated podocyte ablation has been used by Wharram et al. (25) in rats to conclusively demonstrate that podocyte loss causes glomerulosclerosis in adults. The system we use differs from that of the system of Wharram et al. in a number of ways. First, in the system of Wharram et al., podocyte ablation is achieved by the administration of DTA (from 500–50,000 ng/kg body wt) to rats expressing the DTA receptor on podocytes. In our systems (both the Podocin-Cre; ROSA-DTA model and the doxycyclin-inducible model), podocyte-specific expression of Cre ensures podocyte-specific production of DTA and the subsequent suicide of these cells. The internal production of DTA eliminates the need for handling DTA, which has a lethal dose of ∼100 ng of toxin/kg body wt for humans. Second, the system we use was developed in mice, whereas the system of Wharram et al. is in rats. There are advantages in using rats, and there are also advantages in using mice. A possible application of the inducible system in mice is to produce partial podocyte ablation and proteinuria in a given genetically altered mouse strain to assess whether the gene in question influences the progression of proteinuria. The large collection of mutant mice and the availability of a variety of other genetic tools may aid such efforts. Last, both the Podocin-Cre;ROSA-DTA system and the doxycyclin-inducible system work efficiently for embryonic ablation of podocytes (Figs. 1–6 and data not shown). While the system in rats has been successfully applied in adult rats to generate podocyte loss, the efficiency of maternally administered DTA on fetal podocytes has not yet been demonstrated.
Podocyte disruption during nephrogenesis affects the development of the filtration barrier and overall glomerular organization before massive cell death.
It may be predictable that podocyte loss results in severe interruption of glomerulogenesis. However, it appears that glomerulogenesis is affected at the ultrastructural level even before overt podocyte apoptosis (Fig. 4). Since DTA production starts after the Podocin promoter becomes active, there is no direct effect on podocytes before the late S-shaped stage when PODOCIN is first expressed. A delay between activation of the Podocin promoter and DTA-mediated cell death is inevitable due to the multiple intermediate steps involved. It appears that the affected podocytes lose their signaling ability, due to translational arrest, long before their structural disintegration. As an apparent result of such signaling loss, endothelial cells had reduced fenestration before overt podocyte apoptosis (Fig. 4).
Podocyte disruption alters cell-cell interactions.
Nephrogenesis is orchestrated by physical interactions and signaling interchanges among major progenitor cell populations. Early glomerular structures in the outer cortex showed no obvious abnormalities in mutants. On one hand, this reflects the fact that Podocin-Cre becomes active only after the late S-shaped stage. On the other hand, it implies that there are no long-range effects from more mature and deeply seated structures to the development of the emerging structures in the outer cortex. This only considers the potential effects of growth factors, cytokines, and other secreted factors involved in long-distance signaling. Eventual functional loss of the glomeruli would certainly cause secondary damage to the entire organ. In addition, we did not observe significant variation in the proliferation rate of PAX2- or WT1-positive cells, implying an absence of compensatory proliferation in these major nephron progenitor populations. Invasion of the cleft of S-shaped glomeruli by angioblasts appears to be normal. However, translational arrest and apoptosis of the podocytes reduces VEGF expression dramatically. VEGF secreted from podocytes plays a key role in normal development of the glomerular vasculature (7). Dramatic VEGF decrease in mutant mice disrupts the cross talk between podocytes and endothelial cells, leading to decreased fenestration and GBM abnormalities.
Effects of podocyte loss beyond the glomerulus.
Developmental podocyte damage resulted in abnormalities in structures outside the glomerulus. Compromised filtration caused leakage of blood cells into Bowman's capsule and tubules. Further disintegration of the glomerulus may cause hemorrhage in nearby areas. Some of the interstitial hemorrhagic areas are in the medulla, far away from glomeruli, and are unlikely to be caused directly by compromised glomerular vasculature. We postulate that such medullary interstitial hemorrhages may be due to one or more potential causes. First, podocyte ablation interrupts VEGF signaling within the kidney and irreversibly affects development and maintenance of the renal vasculature beyond the glomerular vasculature. Besides the reduction in VEGF signals, podocyte destruction may also cause release of cytokines that may affect the development or maintenance of the renal vasculature. Alternatively, medullary interstitial hemorrhages may also be caused by hemodynamic changes due to the defective filtration barrier and later the disintegration of the glomerulus.
Initial establishment of nephron segments is achieved despite ongoing disruption of the podocytes. However, structural and molecular anomalies were observed in the nephron, with the proximal tubule being affected the most. Average lumen diameter was greatly reduced in most proximal tubules, with the exception of a few that had dilated lumens. Mutant proximal tubule epithelial cells also showed a significantly increased apoptosis rate. While altered signal communication between the glomerulus and the proximal tubule might contribute to these changes (11), it is also possible that the urodynamic changes, concurrent proteinuria, or hematuria were the more important causes. The reduction of AQP2 expression in the mutant collecting duct principal cells is most likely caused by urodynamic changes. The reduction of urine production is consistent with the near absence of mature functional glomeruli in the mutant mice, based on morphological criteria. The small amount of urine produced is likely mostly from the developing glomeruli that are in the process of destruction. Alternatively, some of the fluid recovered from the bladder was produced by tubules.
GRANTS
Urinalysis was performed in the Renal Disease Model Core within the George M. O'Brien Washington University Center for Kidney Disease Research (NIHP30DK079333). Electron microscopic images were acquired from the core facility at Washington University School of Medicine, Department of Otolaryngology, Research Center for Auditory and Visual Studies (NIH P30DC004665). F. Chen was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant R01-DK-067386 and a March of Dimes Award (FY06-343). J. H. Miner was supported by NIDDK Grants R01-DK-064687 and R01-DK-078314 and by an Established Investigator Award from the American Heart Association.
Acknowledgments
We thank Dr. George Jarad for helpful discussions and Jeanette Cunningham for assistance with section preparation for electron microscopy.
Present address of B. W. McDill: Monsanto, St. Louis, MO 63198.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Breitman ML, Clapoff S, Rossant J, Tsui LC, Glode LM, Maxwell IH, Bernstein A. Genetic ablation: targeted expression of a toxin gene causes microphthalmia in transgenic mice. Science 238: 1563–1565, 1987. [DOI] [PubMed] [Google Scholar]
- 2.Breitman ML, Rombola H, Maxwell IH, Klintworth GK, Bernstein A. Genetic ablation in transgenic mice with an attenuated diphtheria toxin A gene. Mol Cell Biol 10: 474–479, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buch T, Heppner FL, Tertilt C, Heinen TJ, Kremer M, Wunderlich FT, Jung S, Waisman A. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods 2: 419–426, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Chang CP, McDill BW, Neilson JR, Joist HE, Epstein JA, Crabtree GR, Chen F. Calcineurin is required in urinary tract mesenchyme for the development of the pyeloureteral peristaltic machinery. J Clin Invest 113: 1051–1058, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen F, Capecchi MR. Paralogous mouse Hox genes, Hoxa9, Hoxb9, and Hoxd9, function together to control development of the mammary gland in response to pregnancy. Proc Natl Acad Sci USA 96: 541–546, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collier RJ Understanding the mode of action of diphtheria toxin: a perspective on progress during the 20th century. Toxicon 39: 1793–1803, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 111: 707–716, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutson MR, Kirby ML. Neural crest and cardiovascular development: a 20-year perspective. Birth Defects Res C Embryo Today 69: 2–13, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Jarad G, Cunningham J, Shaw AS, Miner JH. Proteinuria precedes podocyte abnormalities inLamb2−/− mice, implicating the glomerular basement membrane as an albumin barrier. J Clin Invest 116: 2272–2279, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia Q, McDill BW, Li SZ, Deng C, Chang CP, Chen F. Smad signaling in the neural crest regulates cardiac outflow tract remodeling through cell autonomous and non-cell autonomous effects. Dev Biol 311: 172–184, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kazama I, Mahoney ZX, Miner JH, Graf D, Economides AN, Kreidberg JA. Podocyte-specific deletion of BMP7 leads to growth defect of nephrons with inactivation of P38MAPK. J Am Soc Nephrol 19: 2181–2191, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SW, Cho SH, Oh BS, Yeum CH, Choi KC, Ahn KY, Lee J. Diminished renal expression of aquaporin water channels in rats with experimental bilateral ureteral obstruction. J Am Soc Nephrol 12: 2019–2028, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Lee P, Morley G, Huang Q, Fischer A, Seiler S, Horner JW, Factor S, Vaidya D, Jalife J, Fishman GI. Conditional lineage ablation to model human diseases. Proc Natl Acad Sci USA 95: 11371–11376, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsusaka T, Xin J, Niwa S, Kobayashi K, Akatsuka A, Hashizume H, Wang QC, Pastan I, Fogo AB, Ichikawa I. Genetic engineering of glomerular sclerosis in the mouse via control of onset and severity of podocyte-specific injury. J Am Soc Nephrol 16: 1013–1023, 2005. [DOI] [PubMed] [Google Scholar]
- 15.McDill BW, Li SZ, Kovach PA, Ding L, Chen F. Congenital progressive hydronephrosis (cph) is caused by an S256L mutation in aquaporin-2 that affects its phosphorylation and apical membrane accumulation. Proc Natl Acad Sci USA 103: 6952–6957, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB. Podocyte-specific expression of cre recombinase in transgenic mice. Genesis 35: 39–42, 2003. [DOI] [PubMed] [Google Scholar]
- 17.O'Kane CJ, Moffat KG. Selective cell ablation and genetic surgery. Curr Opin Genet Dev 2: 602–607, 1992. [DOI] [PubMed] [Google Scholar]
- 18.Palmiter RD, Behringer RR, Quaife CJ, Maxwell F, Maxwell IH, Brinster RL. Cell lineage ablation in transgenic mice by cell-specific expression of a toxin gene. Cell 50: 435–443, 1987. [DOI] [PubMed] [Google Scholar]
- 19.Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Pentz ES, Moyano MA, Thornhill BA, Sequeira Lopez ML, Gomez RA. Ablation of renin-expressing juxtaglomerular cells results in a distinct kidney phenotype. Am J Physiol Regul Integr Comp Physiol 286: R474–R483, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Quaggin SE, Kreidberg JA. Development of the renal glomerulus: good neighbors and good fences. Development 135: 609–620, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Saito M, Iwawaki T, Taya C, Yonekawa H, Noda M, Inui Y, Mekada E, Kimata Y, Tsuru A, Kohno K. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nat Biotechnol 19: 746–750, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Shankland SJ The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int 69: 2131–2147, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Soriano P Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21: 70–71, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Wiggins RC The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Wu S, Wu Y, Capecchi MR. Motoneurons and oligodendrocytes are sequentially generated from neural stem cells but do not appear to share common lineage-restricted progenitors in vivo. Development 133: 581–590, 2006. [DOI] [PubMed] [Google Scholar]