Abstract
To facilitate an understanding of injury-induced changes within the nervous system, we used a single-cell, in vitro model of axonal injury. Sensory neurons were individually dissociated from the CNS of Aplysia and placed into cell culture. The major neurite of some neurons was then transected (axotomized neurons). Axotomy in hemolymph-containing culture medium produced long-term hyperexcitability (LTH-E) and enhanced neuritic sprouting (long-term hypermorphogenesis [LTH-M]). Axotomy in the absence of hemolymph induced LTH-E, but not LTH-M. Hemolymph-derived growth factors may activate tyrosine receptor kinase (Trk) receptors in sensory neurons. To examine this possibility, we treated uninjured (control) and axotomized sensory neurons with K252a, an inhibitor of Trk receptor activity. K252a depressed the excitability of both axotomized and control neurons. K252a also produced a distinct pattern of arborizing outgrowth of neurites in both axotomized and control neurons. Protein kinase C (PKC) is an intracellular signal downstream of Trk; accordingly, we tested the effects of bisindolylmaleimide I (Bis-I), a specific inhibitor of PKC, on the axotomy-induced cellular changes. Bis-I blocked LTH-E, but did not disrupt LTH-M. Finally, because Trk activates the extracellular signal regulated kinase pathway in Aplysia sensory neurons, we examined whether this pathway mediates the injury-induced changes. Sensory neurons were axotomized in the presence of U0126, an inhibitor of mitogen-activated/extracellular receptor-regulated kinase. U0126 blocked the LTH-M due to axotomy, but did not impair LTH-E. Therefore distinct cellular signaling pathways mediate the induction of LTH-E and LTH-M in the sensory neurons.
INTRODUCTION
The responses triggered in a neuron by injury depend on intrinsic signals, i.e., signals originating within the injured neuron, and extrinsic signals, i.e., signals derived from the cellular and molecular microenvironment of the injured neuron (Goldberg and Barres 2000). One potential response of neurons to axonal injury is axonal regeneration. Axons in the CNS of higher invertebrates, unlike those in the mammalian CNS (Raff et al. 2002; Schwab 2002), can regenerate after axotomy (Bittner 1991). Axons of central sensory neurons in the marine snail Aplysia exhibit extensive sprouting (hypermorphogenesis) after transection, both in the CNS (Steffensen et al. 1995) and in dissociated cell culture (Bedi and Glanzman 2001; Bedi et al. 1998). Axotomy-induced hypermorphogenesis of Aplysia sensory neurons depends on activation of the cAMP/protein kinase A (PKA) pathway (Bedi et al. 1998). As well as hypermorphogenesis, axotomy triggers hyperexcitability in sensory neurons (Ambron et al. 1996; Bedi et al. 1998; Gunstream et al. 1995; Ungless et al. 2002; Walters et al. 1991). Although PKA activation is necessary for the expression of long-term hyperexcitability (LTH-E) in axotomized sensory neurons (Bedi et al. 1998; Liao et al. 1999), the induction of LTH-E appears to depend on other protein kinases as well, particularly protein kinase G (PKG; Lewin and Walters 1999; Sung et al. 2004). Protein kinase C (PKC) has also been implicated in LTH-E in Aplysia sensory neurons (Manseau et al. 1998), but the precise role of PKC in injury-related changes is currently unknown.
Mitogen-activated protein kinase (MAPK), which is downstream from PKG in Aplysia sensory neurons, may also play a critical role in LTH-E. Intrasomatic injection of vertebrate extracellular signal-regulated kinase 1 (ERK1) into Aplysia sensory neurons induces LTH-E (Sung et al. 2001). Furthermore, endogenous MAPK is activated by crushing nerves that contain the axons of sensory neurons and Aplysia MAPK is a target of PKG (Sung et al. 2004). Until now, work on the role of ERK/MAPK signaling in injury-induced changes in Aplysia sensory neurons has used sensory neurons in vivo. This work has involved nerve crush, in which the axons not only of sensory neurons, but also of other neurons, have been damaged. Using this protocol it is difficult to assess whether ERK activation after nerve injury is induced by signals intrinsic to the sensory neurons or, rather, by extrinsic modulatory factors released from injured nonsensory neurons (or from nonneuronal cells). We have used an in vitro, single-cell model of axonal injury (Ambron et al. 1996; Bedi et al. 1998) to study the role of ERK, as well as other kinases, in injury-induced changes in sensory neurons. This model permits rigorous dissection of the contribution of intrinsic and extrinsic signals to long-term changes caused by axotomy. Here, we have examined the potential roles of ERK, Trk, and PKC in both LTH-E and LTH-M. We find that the roles of these kinases in injury-induced electrophysiological changes in sensory neurons differ from their roles in injury-induced morphological changes. Some of our results have been previously published in abstract form (Bedi and Glanzman 2002).
METHODS
Animals
Adult Aplysia californica (75–125 g) were obtained from local suppliers (Alacrity Marine, Redondo Beach, CA; and M-REP, Escondido, CA). Animals were housed in a 50-gal aquarium filled with cooled (14°C) aerated artificial seawater (ASW; Instant Ocean, Aquarium Systems, Mentor, OH). All animals were housed for ≥24 h before being used for cell culturing.
Cell cultures
Mechanosensory neurons of Aplysia were individually dissociated from pleural ganglia and placed into dissociated cell culture as described previously (Bedi and Glanzman 2001; Bedi et al. 1998). The cultures contained only isolated sensory neurons; they did not contain either interneurons or glia. After the neurons were cultured, the culture dishes were placed into a cell culture incubator (Model No. 3920; Thermo Forma, Marietta, OH), which was kept at 18°C. All cultures were 2 days old at the start of the experiments. Therefore on experimental Day 1 the neurons had been in culture for 48 h.
Electrophysiology and assessment of morphology
All experiments were performed at room temperature (20–22°C). Before the start of the experimental session on Day 1 the hemolymph-containing culture medium (Schacher and Proshansky 1983) was replaced with perfusion medium (50% sterile ASW/50% Liebowitz-15 [L-15; Sigma, St. Louis, MO]) plus appropriate salts. The perfusion medium was perfused through the culture dishes at 0.4 ml/min beginning 30 min prior to, and continuing throughout, electrophysiological and morphological assessments. The purpose of the extensive perfusion prior to the onset of the experiments was to eliminate, as far as possible, the effects of hemolymph and its soluble factors. Electrophysiological and morphological measurements were made on the same neurons. The excitability of the sensory neurons was assessed by injecting a 2-s pulse of positive current into the neurons using a sharp electrode (15–30 MΩ). Two levels of current, 1 and 2 nA, were used. All of the reported results were for the tests with 2 nA. (The results for 1 nA of current were substantially similar.) Spike threshold was determined by injecting 30-ms pulses of positive current of increasing magnitude into the neurons. In addition, the branches on the neurites of each neuron were counted and the length of the major neurite (axonal length) was measured. Axonal length was determined to be the length of the major neurite measured from the point at which the neurite arose from the cell body to the end of the neurite's longest branch (see Bedi and Glanzman 2001 for additional information regarding the methods used for electrophysiological and morphological assessment). Following electrophysiological and morphological assessment on Day 1 of the experiment some of the neurons in each culture dish were axotomized with a glass microelectrode (Axotomized neurons). After the axon was transected, the distal segment of the Axotomized neuron was removed. Other sensory neurons in the culture dish were left intact (Control neurons). The ASW/L-15 perfusion medium in the cell culture dishes was then replaced with hemolymph-containing culture medium in some of the experiments; in other experiments the cell cultures were left in the perfusion medium. Afterward all culture dishes were returned to the 18°C incubator; 24 h later the electrophysiological properties and morphology of the Axotomized and Control neurons were reassessed. In addition, we also measured the spike threshold, membrane potential, membrane resistance, and axonal length of the neurons. Results of these measurements are presented in Tables 1–5.
TABLE 1.
Additional data about electrophysiological and morphological changes in Control and Axotomized neurons during Days 1 and 2 for the experiments in defined control culture medium
| L-15/ASW | ||||||
|---|---|---|---|---|---|---|
| Parameter | Control |
Axotomized | ||||
| Day 1 | Day 2 | P | Day 1 | Day 2 | P | |
| Spike threshold, nA | 0.5 ± 0.08 | 0.7 ± 0.14 | NS | 0.7 ± 0.06 | 0.5 ± 0.04 | <0.01 |
| Membrane potential, mV | 39.5 ± 5.1 | 40.0 ± 5.2 | NS | 46.4 ± 1.4 | 44.0 ± 1.5 | NS |
| Membrane resistance, MΩ | 47.1 ± 8.7 | 51.6 ± 9.5 | NS | 80.1 ± 25.0 | 61.9 ± 4.9 | NS |
| Axonal length, μm | 604.0 ± 74.0 | 757.0 ± 118.0 | NS | 720.0 ± 75.0 | 832.0 ± 128.0 | NS |
Values are means ± SE for spike threshold (measured with a 30-ms pulse of depolarizing current), resting membrane potential, and membrane resistance (measured with a 0.1-nA, 30-ms hyperpolarizing pulse). Axotomy produced a significant decrease in spike threshold. There were no other significant electrophysiological or morphological changes in the two groups of neurons over the 24 h of the experiments. Significances of the electrophysiological and morphological changes in this and the following tables were assessed with paired t-tests. NS, not significant.
TABLE 5.
Additional data from control experiments performed in 0.1% DMSO
| Culture Medium (DMSO) | ||||||
|---|---|---|---|---|---|---|
| Parameter | Control |
Axotomized | ||||
| Day 1 | Day 2 | P | Day 1 | Day 2 | P | |
| Spike threshold, nA | 0.7 ± 0.07 | 0.6 ± 0.05 | NS | 0.7 ± 0.04 | 0.5 ± 0.04 | <0.0004 |
| Membrane potential, mV | 44.4 ± 1.3 | 42.5 ± 1.0 | NS | 46.4 ± 0.8 | 42.5 ± 0.7 | <0.0005 |
| Membrane resistance, MΩ | 55.3 ± 3.7 | 59.6 ± 4.4 | NS | 52.9 ± 3.8 | 63.1 ± 3.1 | <0.04 |
| Axonal length, μm | 712.0 ± 68.0 | 884.0 ± 105.0 | <0.02 | 791.0 ± 68.0 | 998.0 ± 69.0 | <0.002 |
There were significant decreases in spike threshold and in membrane potential, as well as a significant increase in membrane resistance, in the Axotomized group. Also, there were significant increases in axonal length in both groups.
Note that factors such as the time of year and the state of the animals from which the sensory neurons are dissociated can also influence the excitability of the neurons. In particular, during the late summer wild Aplysia in the Pacific Ocean become reproductively mature prior to dying. Neurons dissociated from such animals tend to be hypoexcitable. Therefore we did not use animals caught in the wild during late summer, nor did we use hemolymph from such animals for cell culture medium.
Application of kinase inhibitors
In some experiments cultured sensory neurons were treated with the protein kinase inhibitor K252a (Calbiochem, San Diego, CA) on Day 1 of the experiments. The inhibitor was initially dissolved in 18 MΩ water (DH2O) and then added to ASW/L-15 perfusion medium. The K252a was perfused into cell culture dishes 30 min prior to the time at which some neurons were axotomized. (Note that the K252a-treated culture dishes also contained Control neurons that were left unaxotomized.) Then the perfusion was stopped, some of the cells were axotomized, and the K252a (200 and 50 nM) was left in the culture dishes for an additional 2.5 h. At the end of this incubation period the inhibitor was washed out of the dishes with hemolymph-containing cell culture medium and the dishes were returned to the 18°C incubator until electrophysiological and morphological reassessment the next day (experimental Day 2). In a second experiment using the K252a, the drug was perfused into the culture dishes 10 min after axotomy; the drug was then left in the dishes for another 2.5 h. At the end of this period the inhibitor was washed out of the dishes with hemolymph-containing cell culture medium and the dishes were returned to the incubator. Otherwise, the experimental protocol was identical to that in the experiments in which K252a was applied before axotomy.
Bisindolymaleimide I (Bis-I; Calbiochem, San Diego, CA), a specific PKC inhibitor, was initially made up as a 5 mM solution in 100% dimethylsulfoxide (DMSO) and then further diluted in DH2O into a stock solution containing 20 μM of Bis-I in 0.4% DMSO. For the experiments 0.1 ml of the stock solution was added to 10 ml perfusion medium (50% L-15/50% ASW), yielding a solution that contained 200 nM Bis-I in 0.004% DMSO.
The MEK inhibitor U0126 (Cell Signaling, Beverly, MA) drug was dissolved in perfusion medium containing 0.1% DMSO and perfused into cell culture dishes.
Statistics
Within-group statistical comparisons (Day 1 vs. Day 2) were made using paired t-test. Between-group comparisons were made using a nonparametric test (Mann–Whitney U test) due to significant inhomogeneity in the group variance, as assessed with Bartlett's tests (Bedi and Glanzman 2001; Bedi et al. 1998). All reported levels of statistical significance represent two-tailed values unless otherwise indicated.
As in our previous studies, we observed that both Control and Axotomized neurons can exhibit significant changes in excitability and morphology from Day 1 to Day 2. Some of these changes, particularly those in the Control neurons, appear to be due not to acute axotomy in vitro but, rather, to the axotomy that occurred during the original dissociation of the neurons from the CNS (see the discussion and also Bedi et al. 1998). Therefore to statistically separate the effects of acute axotomy from the ongoing effects of axotomy during original dissociation, we compare the amount of change from Day 1 to Day 2 in the Control and experimental groups (the Day 2 − Day 1 score).
RESULTS
Axotomy in the absence of hemolymph causes LTH-E but not LTH-M in sensory neurons in vitro
The ERK/MAPK signaling pathway can be activated in vertebrate neurons by the binding of neurotrophins with their postsynaptic receptors (Chao 2003). Aplysia hemolymph contains as yet unidentified growth factors required for the survival and neuritic outgrowth of the sensory neurons in dissociated cell culture (Schacher and Proshansky 1983). In our previous studies of the long-term effects of axotomy (Bedi and Glanzman 2001; Bedi et al. 1998) sensory neurons were placed into hemolymph-containing cell culture medium immediately after dissociation and remained in this medium for 2 days prior to the start of the experiments. Following electrophysiological and morphological assessment on Day 1 of the experiments—after which some of the neurons were axotomized—the neurons were returned to hemolymph-containing culture medium; they remained in the hemolymph for 24 h until electrophysiological and morphological reassessment on Day 2. This raises the question of the extent to which hemolymph-derived growth factors are critical to LTH-E and LTH-M. Based on the results from experiments using isolated sensory neurons in dissociated cell culture, Ambron et al. (1996) reported that hemolymph is not required for the induction of LTH-E. We sought to confirm this finding and also compare axotomy-induced LTH-E to LTH-M with respect to the requirement for hemolymph-derived growth factors.
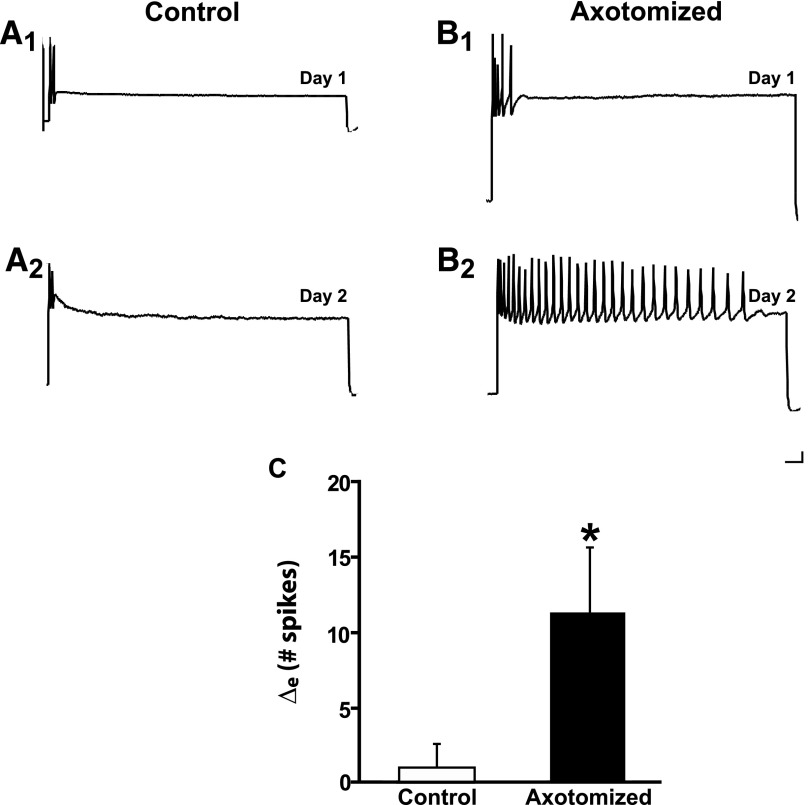
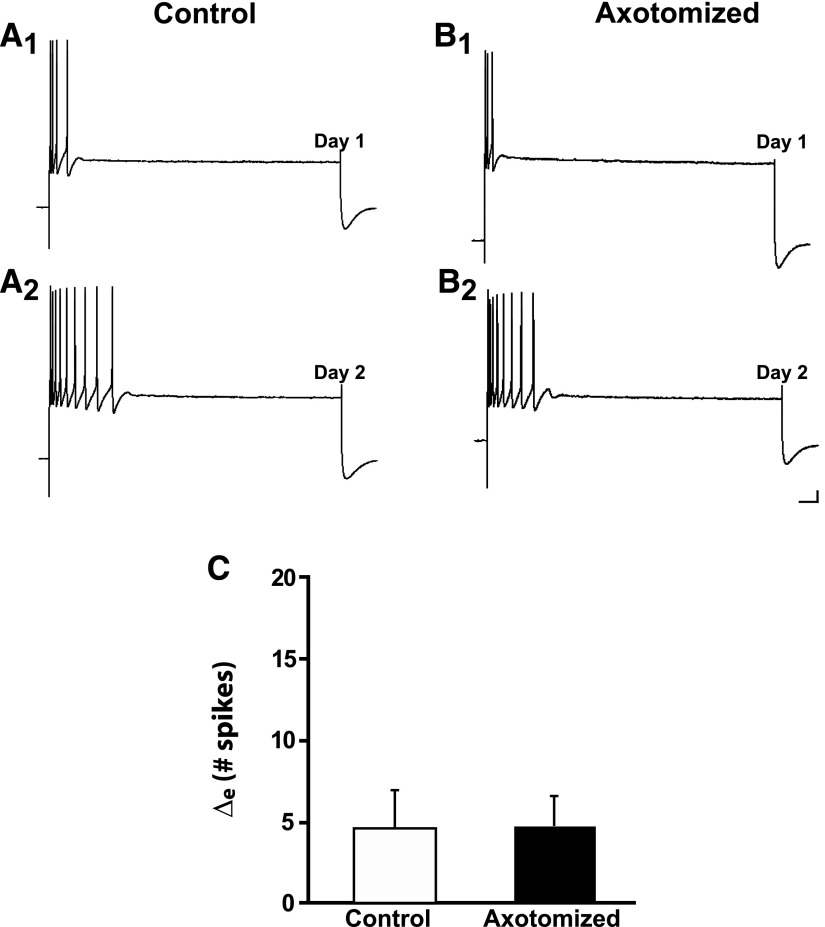
Sensory neurons in dissociated cell culture were axotomized in defined culture medium that did not contain hemolymph and then left for 24 h in hemolymph-free medium (methods). Despite the ostensible absence of hemolymph (discussion), axotomy produced LTH-E in sensory neurons (Fig. 1A). The mean number of spikes elicited by a 2-s depolarizing current pulse in Axotomized neurons was 4.6 ± 0.8 on Day 1 and 15.9 ± 4.1 on Day 2 (n = 10, P < 0.03). Control (unaxotomized) neurons did not exhibit a significant increase in excitability from Day 1 to Day 2. The number of spikes elicited in the Control neurons was 7.3 ± 1.6 on Day 1 and 8.0 ± 3.1 on Day 2 (n = 8, NS). [The difference in the number of spikes elicited by the current pulse on Day 1 in Axotomized and Control neurons was not significant (P > 0.2).] We statistically compared the change in excitability in the Control and Axotomized groups by determining an excitability difference score (the mean of the [Day 2 − Day 1] difference in number of spikes). The excitability difference score (Δe) was 11.3 ± 4.4 spikes for the Axotomized group, which was significantly greater than that of the Control group (0.8 ± 1.9 spikes, P < 0.04; Fig. 1C). These results indicate that hemolymph is not required for axotomy-induced LTH-E in sensory neurons and thus confirm the results of Ambron et al. (1996). In addition to measuring neuronal excitability, we also measured the spike threshold, membrane potential, and membrane resistance of the neurons. (The results from these measurements for this and other experiments in our study are presented in Tables 1–5.) We found that spike threshold decreased significantly in the Axotomized neurons, but not in the Control neurons. There were no significant changes in either membrane potential or membrane resistance (Table 1). Note that the lack of an increase in the input resistance of the Axotomized neurons permits us to rule out a trivial potential explanation for the increase in excitability in this group, i.e., that it was due to a decrease in the cell surface, resulting in an increase in applied current density and hence greater effectiveness of the test stimuli.
FIG. 1.
Axotomy in the absence of the hemolymph induces long-term hyperexcitability (LTH-E) of isolated sensory neurons in culture. A1: representative response of a Control neuron to a 2-s depolarizing current pulse (2 nA in this and subsequent figures) on Day 1 of the experiment. A2: response of the same Control neuron in A1 on Day 2. B1: representative response to depolarizing current pulse on Day 1 of an Axotomized neuron, prior to axotomy. B2: response of the same Axotomized neuron in B1 on Day 2. Notice the significant increase in repetitive firing (decreased accommodation) 24 h after axotomy. Calibration bars in this and subsequent figures: 10 mV, 125 ms. C: change in number of spikes from Day 1 to Day 2 in Control and Axotomized groups. The difference between the 2 groups was significant, as indicated by the asterisk. Graph in this and subsequent figures shows means ± SE.
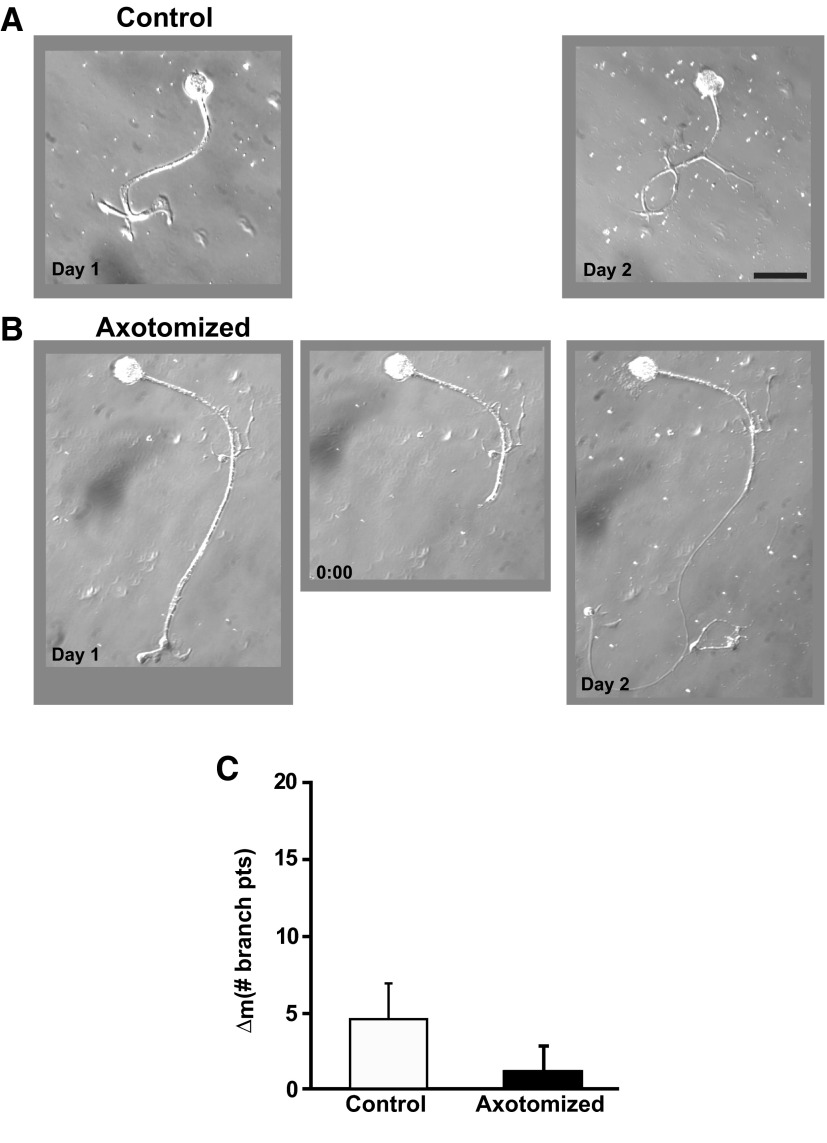
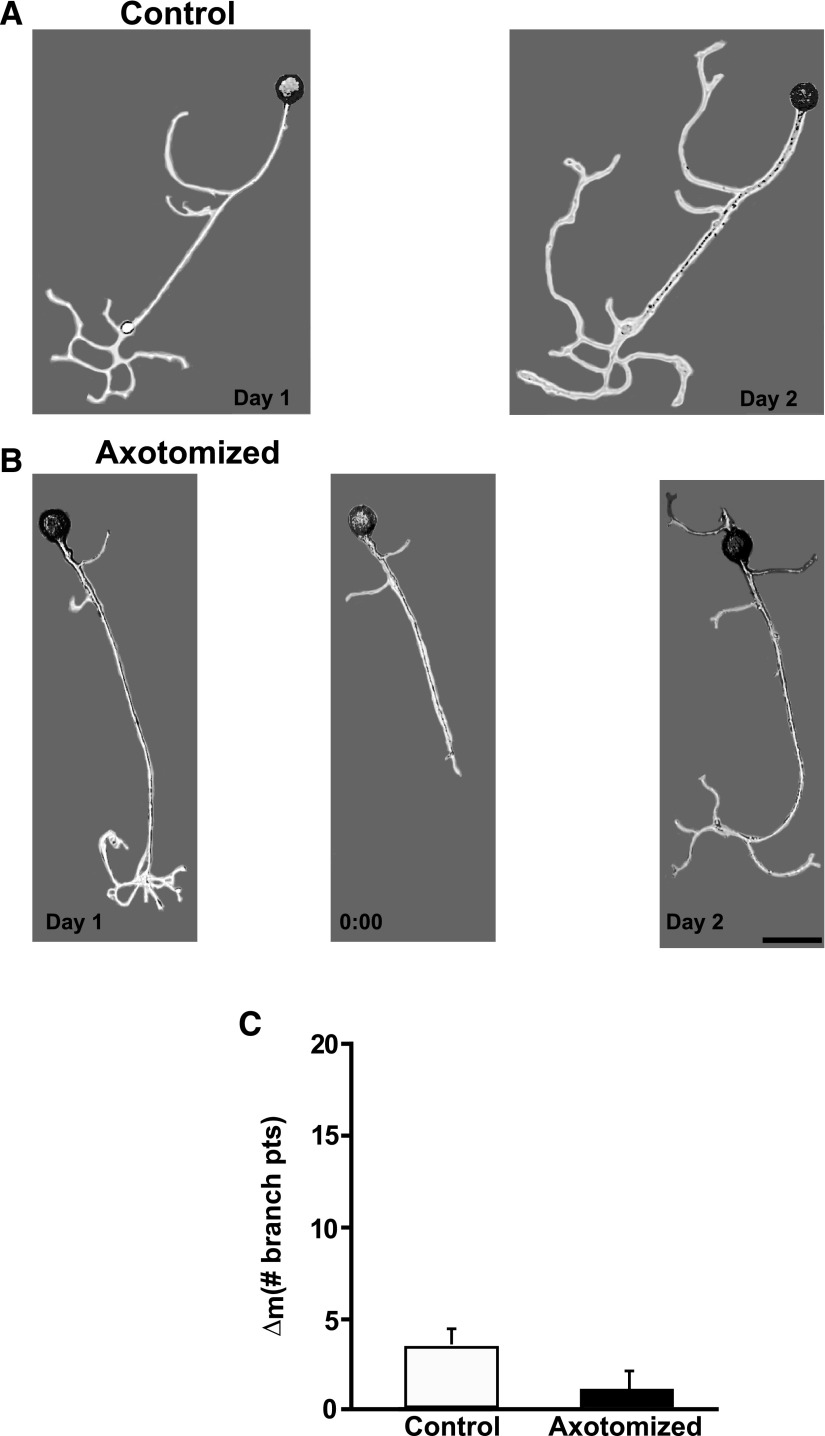
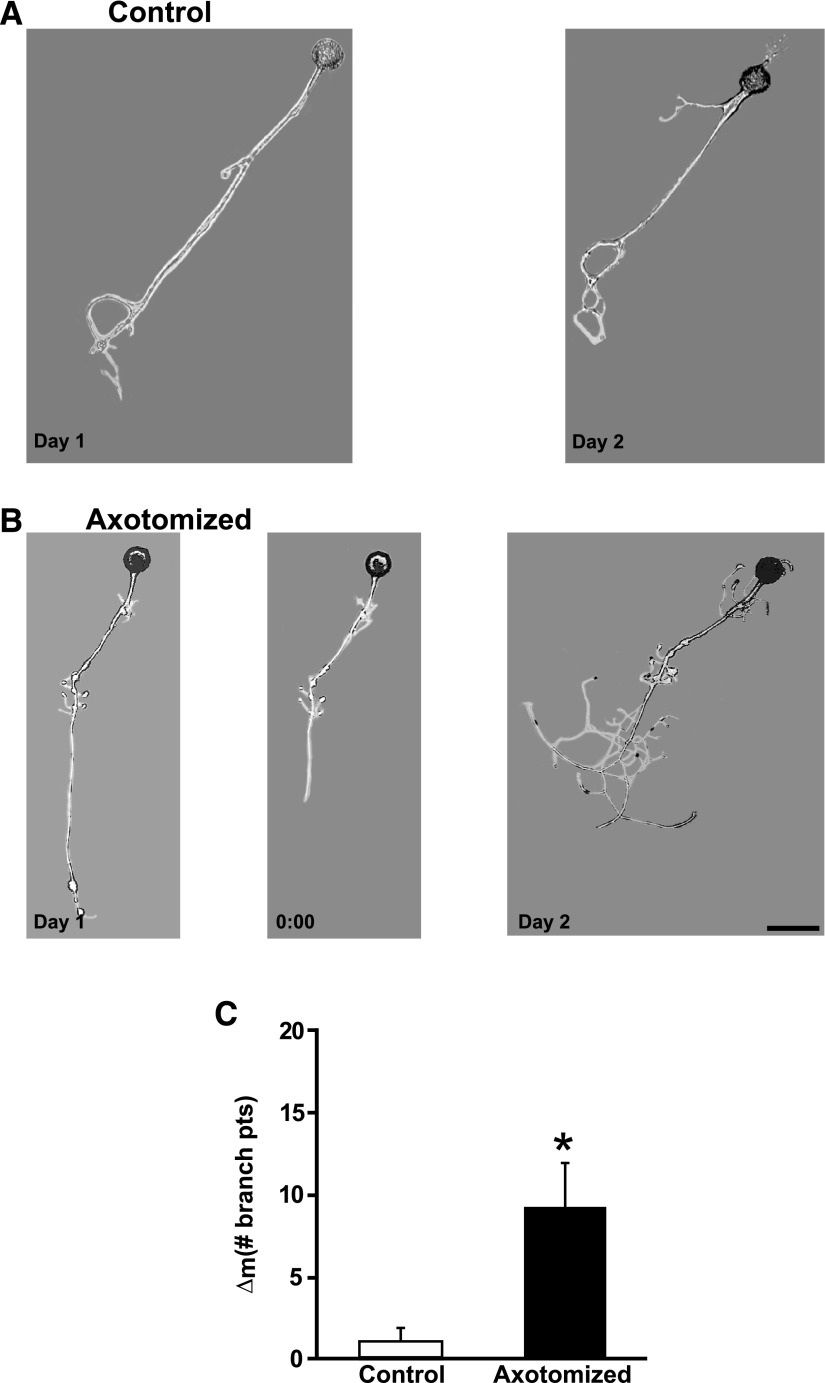
In contrast to axotomy-induced LTH-E, LTH-M did require hemolymph-derived growth factors. In previous experiments—during which hemolymph was present in the cell culture medium—we observed that, whereas there was an increase in the number of neuritic branches over the two experimental days in both Axotomized and Control neurons, the structural change was significantly greater in Axotomized neurons (Bedi and Glanzman 2001; Bedi et al. 1998). [See also the data from the experiments Axotomized and Control neurons in DMSO-containing medium (Fig. 9).] With hemolymph ostensibly absent from the culture medium, however, we did not observe a significant difference between the Axotomized and Control neuron with respect to their structural changes (Fig. 2, A and B). The mean number of neuritic branches in Control neurons was 3.0 ± 1.3 on Day 1 and 7.9 ± 2.6 on Day 2, a nonsignificant change (P > 0.08). The mean number of neuritic branches in Axotomized neurons significantly increased from Day 1 (3.3 ± 0.6) to Day 2 (9.2 ± 1.5; P > 0.03). However, the mean change in number of neuritic branches from Day 1 to Day 2 (morphological difference score [Δm]) for the Axotomized neurons (5.9 ± 1.4) was not statistically different from the Δm for the Control neurons (4.9 ± 2.4, P > 0.3; Fig. 2C).
FIG. 2.
Axotomy in the absence of hemolymph does not induce long-term hypermorphogenesis (LTH-M). A: photomicrographs of a Control neuron on Day 1 and the same neuron on Day 2. B: photomicrographs of an Axotomized neuron on Day 1, prior to axotomy (left), the same neuron immediately after axotomy on Day 1 (0:00 h, middle), and the neuron 24 h later on Day 2 (right). Scale bar for this and subsequent micrographs, 100 μm. C: change in the number of branch points from Day 1 to Day 2 for the Axotomized and Control groups. There was no significant difference between the 2 groups.
We also examined a second morphological parameter: the length of the major neurite of each sensory neuron (axonal length) on Days 1 and 2 (methods). The changes in axonal length generally correlated with those in the number of neuritic branches (see Tables 1–5); however, results from the measures of branch number and axonal length were sufficiently different to suggest that the two measures reflected different underlying cellular processes (discussion).
K252a inhibits the excitability of sensory neurons and also triggers sprouting
The above-cited results suggest that hemolymph-derived growth factors mediate the axotomy-induced long-term morphological changes in Aplysia sensory neurons. One class of growth factors, neurotrophins, has diverse actions on neurons, including effects on cell survival, neuritic outgrowth, and synaptic transmission (Segal and Greenberg 1996). The effects of neurotrophins are mediated by transmembrane-receptor signaling molecules, particularly Trk receptors (Chao 2003). It has been previously shown that inhibition of Trk decreases the baseline excitability of sensory neurons and also blocks the short-term hyperexcitability of sensory neurons due to application of serotonin (5-HT), an endogenous neuromodulatory transmitter (Purcell and Carew 2001). To determine whether Trk receptors might be involved in the long-term effects of injury on in vitro sensory neurons, we used K252a, a potent and relatively specific inhibitor of Trk activity (Angeles et al. 1998; Berg et al. 1992; Watson et al. 2001; but see Kase et al. 1987). K252a has been reported to block activation of the identified endogenous receptor tyrosine kinase ApTrkl within Aplysia sensory neurons (Ormond et al. 2004).
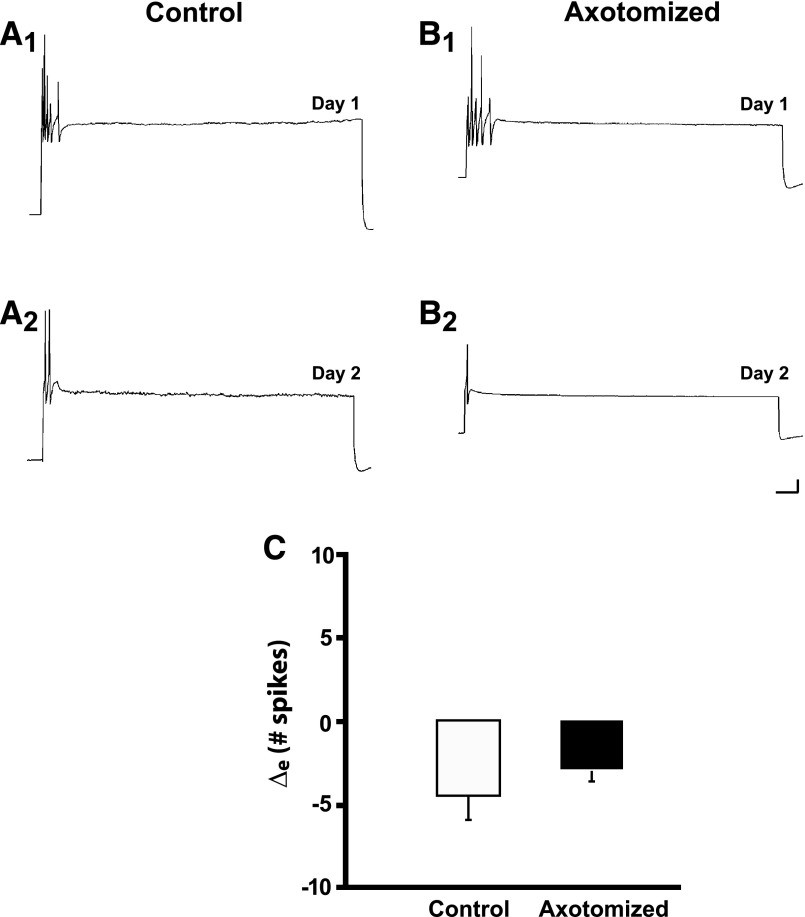
Culture medium containing K252a (200 nM) was perfused into a culture dish 30 min before some of the cells in a culture dish were to be axotomized and then left in the dish for another 2.5 h; afterward the K252a-containing medium was washed out and replaced with cell culture medium containing hemolymph (methods). K252a had striking effects on both the electrophysiological and morphological properties of in vitro sensory neurons. First, in the presence of K252a axotomy did not induce the usual hyperexcitability in sensory neurons (Fig. 3, A and B). Indeed, the number of elicited spikes significantly decreased from Day 1 to Day 2 in both the Axotomized and Control neurons with K252a treatment. The number of spikes elicited in Control neurons was 7.5 ± 2.1 on Day 1 and 3.1 ± 0.4 on Day 2 (n = 9, P < 0.05). The number of spikes elicited in the Axotomized group was 5.7 ± 1.3 on Day 1 and 2.7 ± 0.7 on Day 2 (n = 7, P < 0.01). Injury did not appear to alter the decrease in excitability caused by K252a treatment: the Δe for the excitability changes was −4.4 ± 1.8 spikes in the Control group and −3.0 ± 0.6 spikes in the Axotomized group (P > 0.9, Fig. 3C).
FIG. 3.
The tyrosine receptor kinase (Trk) receptor inhibitor K252a depresses excitability in both Control and Axotomized neurons. A1: response of a Control neuron treated with K252a to a depolarizing current pulse on Day 1. A2: response of the same Control neuron on Day 2. There was a decrease in the number of spikes elicited. B1: response of an Axotomized neuron treated with K252a to a depolarizing current pulse on Day 1. B2: response of the Axotomized neuron on Day 2 (24 h after axotomy). The neuron did not exhibit the hyperexcitability typically observed after axotomy. C: change in number of spikes from Day 1 to Day 2 in Control and Axotomized groups. Both groups exhibited decreased excitability; the difference between the groups was not significant.
To test whether the effects of K252a on axotomy-induced excitability were specific to the concentration of the drug that was used, we performed an additional experiment using a lower concentration of K252a (50 nM). Application of the drug at the lower concentration did not cause a significant change in the excitability of Control neurons, but still blocked axotomy-induced LTH-E in sensory neurons. The mean number of spikes evoked in Control neurons treated with 50 nM K252a was 3.2 ± 0.8 on Day 1 and 4.7 ± 1.9 on Day 2 (n = 10, P > 0.2). The mean number of spikes evoked in Axotomized neurons was 3.9 ± 0.8 on Day 1 and 4.2 ± 0.6 on Day 2 (n = 10, P > 0.7). The Δe values for the two groups were not significantly different (P > 0.8; data not shown). The efficacy of K252a at the lower concentration supports the idea that axotomy-induced LTH-E depends on Trk activity.
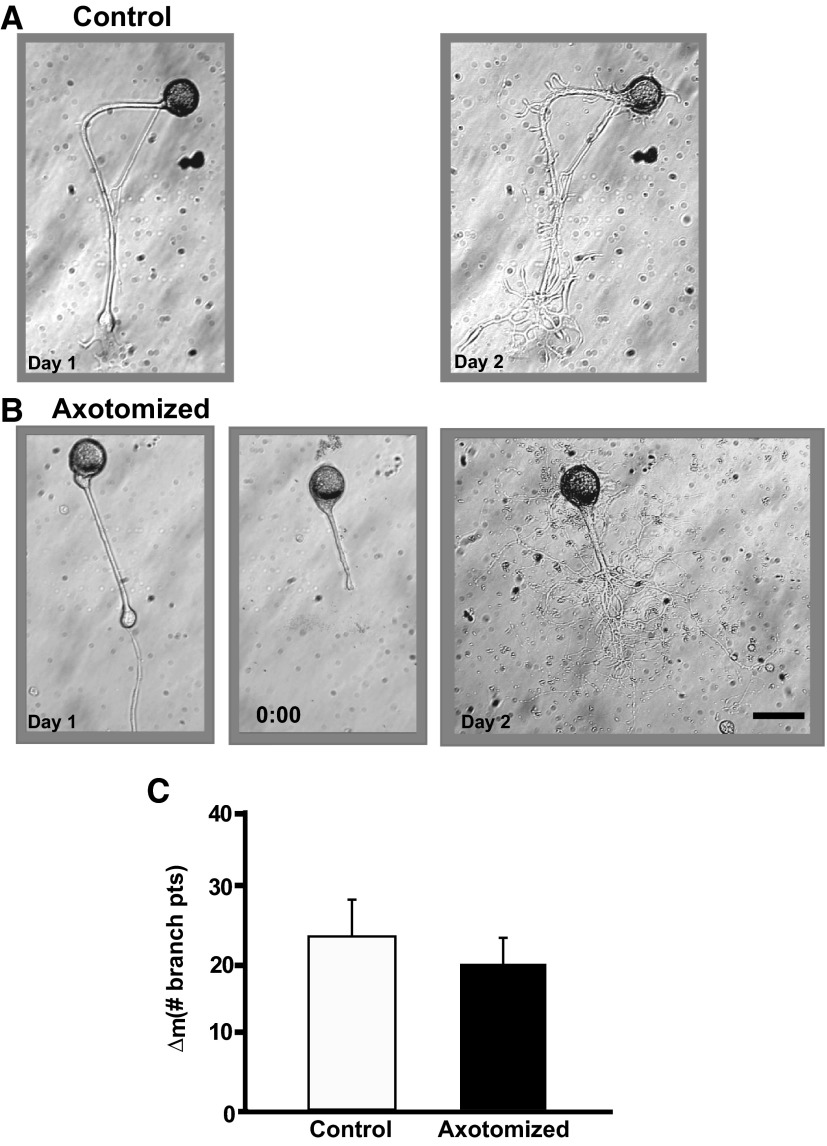
In contrast to its depressive effect on neuronal excitability, K252a had a powerful stimulatory effect on neuritic outgrowth, as indicated by the increase in the number of branches on the neurites of the neurons. [Note that the increase in the number of branches can result either from de novo growth of new branches or from defasciculation of the major neurites of sensory neurons, which actually represent bundles of smaller processes (see Glanzman et al. 1989; Zhu et al. 1994). We did not attempt to distinguish between these two possibilities here (discussion).] The number of branch points increased dramatically from Day 1 to Day 2 in both the Control and Axotomized sensory neurons (Fig. 4, A and B) in the presence of the Trk inhibitor. The number of neuritic branches in Control neurons was 5.4 ± 1.3 on Day 1 and 28.4 ± 6.0 on Day 2 (P < 0.003); the number of neuritic branches in Axotomized neurons was 4.7 ± 1.2 on Day 1 and 23.3 ± 2.0 on Day 2 (P < 0.003). Axotomy did not produce enhanced sprouting/defasciculation of sensory neurites when carried out in the presence of K252a, in contrast to its effect in the absence of the drug (Bedi and Glanzman 1998, 2001). The Δm was not significantly different for the Control and Axotomized groups when K252a was present in the culture dish (P > 0.5, Fig. 3C). This result suggests that axotomy and K252a activate the same, or closely related, signaling pathways in Aplysia sensory neurons, the end effect of which is to stimulate axonal sprouting. The effect on neuronal morphology of applying K252a at a lower concentration (50 nM) was similar to that of the drug at the higher concentration. Specifically, there was a significant increase in the neuritic branching in the Control (15.5 ± 3.0 on Day 1 and 31.1 ± 3.8 on Day 2, P < 0.0001; n = 10) and Axotomized (15.3 ± 2.1 on Day 1 and 29.0 ± 2.4 on Day 2, P < 0.0001; n = 10) neurons. Again, there was no significant difference between the two groups with respect to their Δm values (P > 0.6; data not shown). Interestingly, there was a significant decrease in axonal length following axotomy in K252a (200 nM; Table 2). There was no change in axonal length in the Control group. We address this apparent discrepancy in morphological measures in the discussion.
FIG. 4.
K252a induces exaggerated neuritic outgrowth in both Control and Axotomized neurons. A: photomicrographs of a Control neuron on Day 1 and 24 h later. Note the enhanced outgrowth of the neurites. B: photomicrographs of an Axotomized neuron on Day 1, prior to axotomy (left), the same neuron immediately after axotomy on Day 1 (0:00 h, middle), and the neuron 24 h later on Day 2 (right). The outgrowth of the neurites in this neuron was also enhanced. C: change in the number of branch points from Day 1 to Day 2 for the Control and Axotomized groups. There was no significant difference in neurite outgrowth between the 2 groups.
TABLE 2.
Additional data for Control and Axotomized neurons in experiments that involved application of K252a
| K252a | ||||||
|---|---|---|---|---|---|---|
| Parameter | Control |
Axotomized | ||||
| Day 1 | Day 2 | P | Day 1 | Day 2 | P | |
| Spike threshold, nA | 0.6 ± 0.1 | 1.5 ± 0.7 | NS | 0.5 ± 0.07 | 0.8 ± 0.09 | <0.01 |
| Membrane potential, mV | 45.4 ± 2.0 | 47.0 ± 2.4 | NS | 48.6 ± 2.0 | 50.3 ± 2.8 | NS |
| Membrane resistance, MΩ | 68.8 ± 7.4 | 65.2 ± 6.2 | NS | 67.9 ± 5.1 | 58.9 ± 5.6 | NS |
| Axonal length, μm | 687.0 ± 47.0 | 744.0 ± 43.0 | NS | 728.0 ± 72.0 | 414.0 ± 30.0 | <0.03 |
See legend of Table 1 for details regarding this and subsequent tables. Axotomy with K252a (only the data for the 200 nM experiments are shown) present in the bath produced a significant increase in spike threshold. There were no significant changes in either membrane potential or membrane resistance for the two groups. Although there no significant changes in axonal length in Control neurons, Axotomized neurons exhibited a significant decrease in axonal length.
We also tested whether inhibition of Trk shortly after, rather than prior to, injury would affect the electrophysiological and morphological properties of sensory neurons. K252a (200 nM) was perfused into the cell culture dishes 10 min after the axons of some of the neurons in the dish were severed. Control neurons in the same dishes were also exposed to K252a. The 10-min delay in the addition of K252a did not alter the effect of drug on axotomy-induced excitability; delayed treatment with K252a also blocked LTH-E. The mean number of spikes evoked in Control neurons on Day 1 was 3.4 ± 0.8 and 2.9 ± 0.7 on Day 2 (n = 8, P > 0.5). Furthermore, the number of spikes evoked in the Axotomized group was 4.4 ± 0.6 on Day 1 and 3.9 ± 0.8 on Day 2 (n = 9, P > 0.5; data not shown). Additionally, adding the K252a 10 min after axotomy did not change the stimulatory effect of the inhibitor on the morphology of sensory neurons. There was a significant increase in neuritic branching in both in Control (8.6 ± 2.9 on Day 1 and 22.9 ± 6.3 on Day 2, P < 0.03) and Axotomized (8.1 ± 2.7 on Day 1 and 22.2 ± 3.5 on Day 2, P < 0.002) neurons. However, the Δm values for the two groups did not differ significantly (P > 0.5; data not shown).
Bisindolylmaleimide I, a PKC inhibitor, blocks LTH-E but not LTH-M
PKC has been critically implicated in long-term plasticity (electrophysiological and morphological) (Hu et al. 2007; Kabir et al. 2001; Manseau et al. 1998; Sutton et al. 2004), but its role in injury-related changes has been largely unexamined. To investigate the role of PKC in injury-induced changes Bis-I (200 nM) was perfused into a cell-culture dish 30 min before some of the sensory neurons were axotomized; after 2.5 h the drug was washed out of the dish using normal cell culture medium containing hemolymph. Bis-I, a specific inhibitor of PKC (Toullec et al. 1991), has been shown to inhibit two isoforms of PKC in Aplysia, Apl I, the classical PKC isoform, and Apl II, the novel PKC isoform (Sossin 2007; and WS Sossin, personal communication). In addition, Bis-I has been used previously to examine PKC-dependent morphological and electrophysiological changes in Aplysia neurons (Hu et al. 2007; Kabir et al. 2001; Sutton et al. 2004). We observed that the excitability of Control neurons increased significantly from Day 1 to Day 2 (Fig. 5A). The mean number of spikes elicited from Control neurons increased from 5.0 ± 1.0 on Day 1 to 9.8 ± 2.8 on Day 2 (n = 13, P < 0.05). The enhancement of excitability in the unaxotomized Control neurons cannot be attributed to the presence of Bis-I; we have previously observed a significant increase in the excitability of Control neurons in some experiments (see, e.g., Fig. 1 of Bedi et al. 1998) in which they were exposed to culture medium alone. We believe this increase is due to the ongoing effects of axonal transection during dissociation of the sensory neurons from the pleural ganglion (also see Ambron et al. 1996).
FIG. 5.
Bisindolylmaleimide I (Bis-I), a protein kinase C (PKC) inhibitor inhibits LTH-E. A1: representative response of a Control neuron to a 2-s depolarizing current pulse on Day 1 of the experiment. A2, response of the same Control neuron in A1 on Day 2. B1: representative response to depolarizing current pulse on Day 1 of an Axotomized neuron, prior to axotomy. B2: response of the same Axotomized neuron in B1 on Day 2. C: change in number of spikes from Day 1 to Day 2 in Control and Axotomized groups. The difference between the 2 groups was not significant.
The excitability of Axotomized neurons increased significantly in the presence of Bis-I (Fig. 5B). The mean number of spikes elicited from Axotomized neurons was 4.8 ± 0.9 on Day 1 and 9.6 ± 2.0 on Day 2 (n = 17, P < 0.001). However, the difference between Control and Axotomized neurons with respect to the changes in excitability was not significantly different (Δe = 4.8 ± 2.2 spikes for the Control group vs. 4.9 ± 1.7 spikes for the Axotomized group, P > 0.9; Fig. 5C). Therefore inhibition of PKC blocks the axotomy-induced enhancement of sensory neuron excitability (Bedi and Glanzman 2001 and Fig. 8 in Bedi et al. 1998).
In contrast to the effect of Bis-I on neuronal excitability, we observed no apparent effect of the PKC inhibitor on neuronal morphology in either Control or Axotomized sensory neurons. There was an increase in the number of branches on the neurites of the Control cells from Day 1 to Day 2 in the presence of Bis-I, although this increase was not quite statistically significant (8.7 ± 2.0 on Day 1 vs. 16.2 ± 4.3 on Day 2, n = 13; P > 0.08; data not shown). Axotomized neurons did exhibit a significant structural change from Day 1 to Day 2 (4.8 ± 0.9 on Day 1 vs. 14.8 ± 2.1 on Day 2, n = 17; P < 0.0002). However, as we have previously found in experiments in which only cell culture medium was present (Bedi and Glanzman 2001; Bedi et al. 1998), the Δm was significantly greater in the Axotomized neurons than that in the control neurons (9.9 ± 2.0 vs. 7.5 ± 3.9; P < 0.05, one-tailed significance). In summary, PKC activity appears to be required for the enhancement of excitability in sensory neurons after injury, but does not seem to play a significant role in mediating the effects of injury on morphology.
MEK1/2 inhibitor U0126 does not affect LTH-E, but blocks axotomy-induced LTH-M
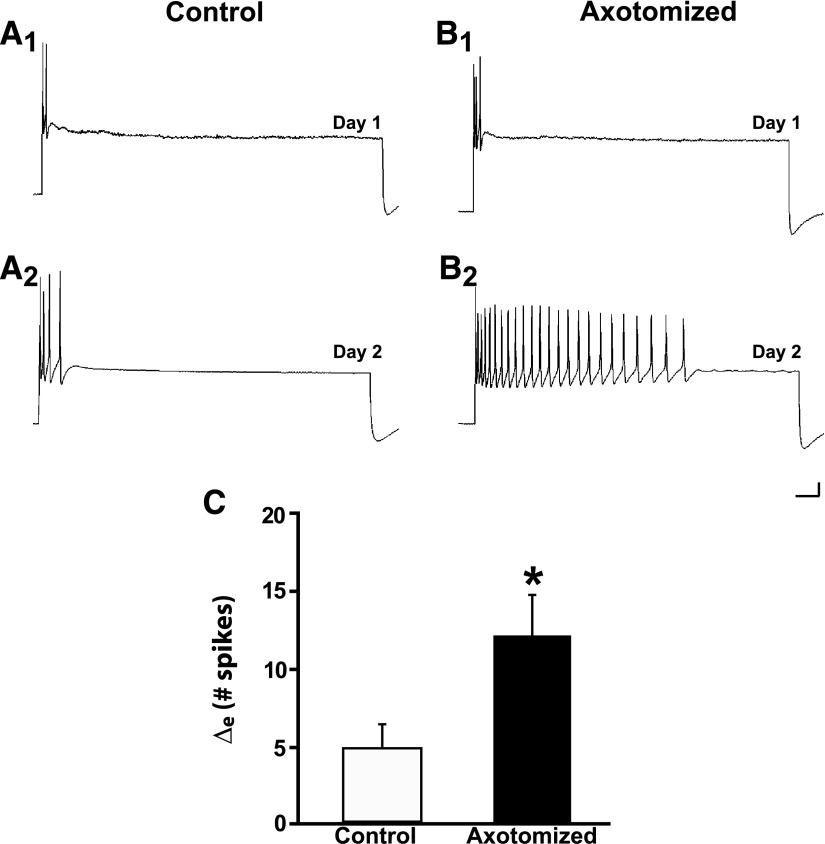
The ERK1/2 kinase pathway has been implicated in signaling mediated by neurotrophin-stimulated Trk receptor activation (Chao 2003). To test whether ERK1/2 signaling plays a role in axotomy-induced long-term changes in the sensory neurons, neurons were treated with U0126, a specific inhibitor of mitogen-activated extracellular signal-regulated kinase (MEK) 1/2, the kinase that phosphorylates ERK1/2 (Davies et al. 2000). The inhibitor (50 μM in 0.1% DMSO) was perfused into a cell-culture dish 30 min prior to axotomy of some of the neurons; the drug was washed out after 2.5 h using normal cell culture medium containing hemolymph. Cell cultures in control experiments were treated identically with culture medium containing 0.1% DMSO with U0126. The Control neurons exhibited a significant increase in excitability from Day 1 to Day 2 (Fig. 6A). The mean number of spikes elicited from Control neurons was 4.3 ± 0.9 on Day 1 and 9.5 ± 2.0 on Day 2 (n = 17, P < 0.005). Neurons that were axotomized in the presence of U0126 also showed an increase in excitability (Fig. 6B). The mean number of spikes elicited in Axotomized neurons was 4.4 ± 0.7 on Day 1 and 16.6 ± 2.4 on Day 2 (n = 21, P < 0.0001). The increase in excitability in the Axotomized neurons was significantly greater than that in the Control neurons. The Δe was 12.9 ± 2.3 spikes for the Axotomized group and 5.2 ± 1.5 spikes for the Control group (P < 0.05, one-tailed significance; Fig. 6C). The enhancement of excitability induced by axotomy in the presence of U0126 was therefore similar to that we obtained when the inhibitor was not present (Fig. 1). Thus MEK1/2 does not appear to contribute to the effect of axotomy on the excitability of Aplysia sensory neurons (see also Sung et al. 2004).
FIG. 6.
U0126, a mitogen-activated/extracellular receptor-regulated kinase (MEK) 1/2 inhibitor, does not block axotomy-induced LTH-E. A1 and A2: responses of a Control neuron on Day 1 and Day 2. There was a modest increase in the number of evoked spikes to the 2-s pulse on the second day. B1 and B2: responses of an Axotomized neuron on Day 1 and Day 2. This neuron exhibited a significant increase in its excitability on Day 2. C: change in number of evoked spikes from Day 1 to Day 2 in Control and Axotomized groups. The difference between the 2 groups was significant, as indicated by the asterisk.
In contrast to its lack of an effect on LTH-E, U0126 inhibited the morphological changes due to acute axonal injury. Control neurons exhibited an increase in neuritic branches from Day 1 to Day 2 in the presence of U0126. The number of branches on the neurites of Control cells was 4.4 ± 0.8 on Day 1 and 7.7 ± 1.3 on Day 2 (P < 0.003, Fig. 7A). Somewhat surprisingly, Axotomized neurons did not show significant changes in their structure from Day 1 to Day 2 when treated with U0126. The number of branches on the neurites of Axotomized cells was 5.5 ± 0.9 on Day 1 and 6.3 ± 1.0 on Day 2 (P > 0.3, Fig. 7B). Although the Control sensory neurons exhibited a greater increase in neuritic branches than the Axotomized neurons in our U0126 experiments, the change in the number of branches [Day 2 − Day 1 (Δm)] between the two groups was not statistically significant. The Δm was 3.4 ± 0.9 branch points in the Control neurons and 0.9 ± 1.0 branch points in the Axotomized neurons (P > 0.2, Fig. 7C).
FIG. 7.
U0126 blocks LTH-M due to acute axotomy. A: photomicrographs of a Control neuron on Day 1 and the same neuron on Day 2. The neuron exhibited a modest increase in the number of neuritic branches. B: photomicrographs of an Axotomized neuron on Day 1, prior to axotomy (left), the same neuron immediately after axotomy on Day 1 (0:00 h, middle), and the neuron 24 h later on Day 2 (right). Treatment with the MEK1/2 inhibitor blocked the increase in the number of branches. C: the change in the number of branch points from Day 1 to Day 2 for the Axotomized and Control groups. The difference between the Control and Axotomized groups was not significant.
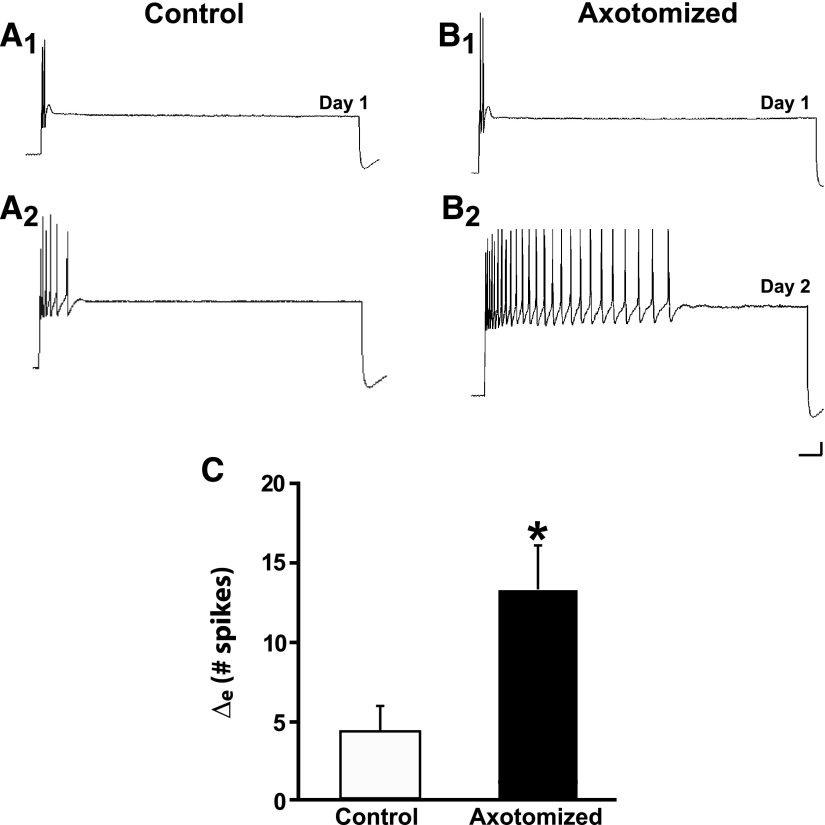
Because DMSO was present in the culture medium in the experiments involving Bis-I and U0126, we assessed the potential effects of this solvent on LTH-E and LTH-M in additional control experiments. In these experiments the cultures were treated with 0.1% DMSO in perfusion medium. Control neurons treated with DMSO exhibited an increase in excitability during the experiment. The number of spikes evoked in the Control neurons treated with DMSO was 5.2 ± 1.7 on Day 1 and 9.2 ± 2.3 on Day 2 (n = 15, P < 0.03; Fig. 8A). Following axotomy in the presence of DMSO, the excitability of sensory neurons also increased significantly (Fig. 8B). The number of spikes evoked in Axotomized neurons was 4.4 ± 0.8 on Day 1 and 18.1 ± 3.1 on Day 2 (n = 18, P < 0.0001). The increase in the excitability in the Axotomized neurons was significantly greater than that in Control neurons: the Δe for Axotomized neurons was 13.7 ± 2.8 spikes, but only 4.0 ± 1.5 spikes for the Control neurons (P < 0.03, Fig. 8C). Therefore sensory neurons axotomized in the presence of DMSO exhibited LTH-E, just as when axotomy was performed in L-15 (Fig. 1). Furthermore, axotomy in DMSO resulted in LTH-M in sensory neurons (Fig. 9, A and B). There was an increase in the number of branches on the neurites of both Control and Axotomized sensory neurons from Day 1 to Day 2 (Controls: 4.3 ± 1.0 branch points on Day 1 vs. 6.1 ± 1.2 on Day 2, P < 0.01; Axotomized: 5.5 ± 1.0 on Day 1 vs. 14.7 ± 2.7 on Day 2, P < 0.004). However, the Δm was significantly greater in the Axotomized neurons (9.2 ± 2.7 branch points) than that in the Control neurons (1.7 ± 0.5 branches, P < 0.005; Fig. 9C). The electrophysiological and morphological results in our DMSO experiments substantially resemble those we have obtained in prior studies (Bedi and Glanzman 2001; Bedi et al. 1998). Therefore DMSO, at the concentration used in our experiments, did not significantly alter the effects of axotomy on the excitability and morphology of sensory neurons.
FIG. 8.
Axotomy induces LTH-E of isolated sensory neurons in culture medium containing 0.1% dimethylsulfoxide (DMSO). A1 and A2: representative responses of a Control neuron treated with DMSO on Day 1 and Day 2. There was a modest increase in excitability in this cell over the 24-h period. B1 and B2: representative responses of an Axotomized neuron treated with DMSO. This neuron exhibited a very large increase in its excitability 24 h after axotomy. C: change in the number of evoked spikes from Day 1 to Day 2 in Control and Axotomized groups. The difference between the 2 groups was statistically significant, as indicated by the asterisk. Note that the results in DMSO resemble those that we have observed earlier in control culture medium without DMSO (Bedi and Glanzman 2001; Bedi et al. 1998).
FIG. 9.
Axotomy induces LTH-M of isolated sensory neurons in culture medium containing 0.1% DMSO. A: photomicrographs of a Control neuron on Day 1 and the same neuron on Day 2. The neuron exhibited a modest increase in outgrowth over the 24-h period. B: photomicrographs of an Axotomized neuron on Day 1, prior to axotomy (left), the same neuron immediately after axotomy on Day 1 (0:00 h, middle), and the neuron 24 h later on Day 2 (right). Axotomy produced a more dramatic increase in outgrowth than was observed in the Control neuron (A). C: the change in the number of branch points from Day 1 to Day 2 for the Axotomized and Control groups. The difference between the 2 groups was statistically significant, as indicated by the asterisk. This pattern of results was like that observed in earlier experiments using culture medium without DMSO (Bedi and Glanzman 2001; Bedi et al. 1998).
DISCUSSION
Different signaling pathways mediate the long-term effects of axotomy on neuronal excitability and structure
We have used a single-cell, in vitro system to investigate the signals that mediate injury-induced changes in neuronal excitability and morphology. Previously, we and others reported that axotomy produces LTH-E and LTH-M of Aplysia sensory neurons in vitro (Ambron et al. 1996; Bedi and Glanzman 2001; Bedi et al. 1998). Here, we have shown that the absence of hemolymph after axotomy, inhibition of Trk receptors, inhibition of PKC, and inhibition of MEK1/2 have disjunctive effects on the excitability and morphology of sensory neurons. Specifically, the absence of hemolymph blocked LTH-M, but not LTH-E; inhibition of Trk receptors reduced the overall excitability of sensory neurons, but stimulated hypermorphogenesis of the neurons in the absence of injury; inhibition of PKC blocked LTH-E, but did not affect LTH-M; and, finally, inhibition of MEK1/2 did not affect LTH-E, but blocked LTH-M. These results indicate that injury activates multiple intracellular signaling cascades within sensory neurons and that the cascades that mediate LTH-E are at least partly distinct from those that mediate LTH-M (Fig. 10).
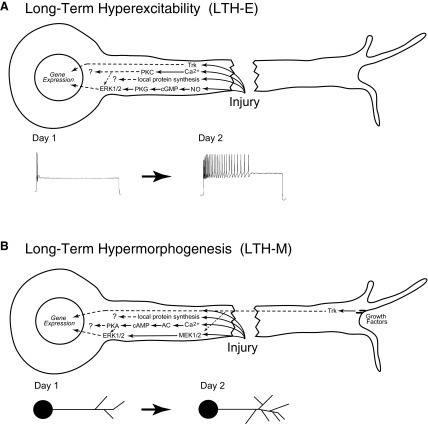
FIG. 10.
Signaling pathways that mediate injury-induced, long-term cellular changes in sensory neurons. A: model for LTH-E following axotomy. Axonal injury causes activation of nitric oxide (NO) synthase, thereby leading to elevation of NO within the axon; enhanced levels of NO, in turn, activate the cyclic guanosine monophosphate (cGMP)–dependent protein kinase G (cGMP–PKG) pathway (Lewin and Walters 1999). PKG activates extracellular signal regulated kinase (ERK)1/2, which can then translocate to the nucleus to regulate gene expression (Sung et al. 2004). In the case of LTH-E, ERK1/2 activation does not appear to result from MEK activity (Fig. 5). In addition, axotomy also leads to stimulation of a Trk-dependent pathway, which also appears to be required for LTH-E (Fig. 3). The injury-induced stimulation of the Trk-dependent pathway must be downstream of Trk receptors because growth factors are not required for LTH-E (Ambron et al. 1996; and Fig. 1). Trk receptors can also translocate to the nucleus and regulate gene expression (Riccio et al. 1997). Influx of Ca2+ after injury leads to activation of PKC (Fig. 5), which can also interact with ERK1/2 (Kawasaki et al. 2004). Ongoing activity in the Trk-dependent pathway is also required for noninjury-dependent excitability (Fig. 3). Local protein synthesis (Weragoda and Walters 2007; Weragoda et al. 2004) may well contribute to LTH-E as well, but we do not yet know which proteins are locally synthesized due to injury. B: model for long-term hypermorphogenesis (LTH-M) following axotomy. Injury results in an influx of Ca2+, which activates adenylyl cyclase (AC), leading to synthesis of cAMP and activation of protein kinase A (PKA), which is required for the induction of LTH-M (Bedi et al. 1998). PKA also modulates ERK1/2 (Sweatt 2004), which can then translocate to the nucleus and regulate gene expression. In addition to its activation by PKA, ERK1/2 is also likely to be activated by MEK1/2 after injury (Zhao et al. 2007); MEK1/2-dependent activation of ERK1/2 is critical for injury-induced LTH-M (Fig. 7). As well as MEK1/2 activity, injury-induced LTH-M depends on growth factors (Fig. 2). The effect of growth factors on the morphology of sensory neurons is likely to depend on Trk receptors, particularly nork (Hislop et al. 2004). Trk receptors may also stimulate activation of MEK1/2 (Chao 2003). In addition, local protein synthesis and its products may contribute to the injury-induced morphological changes. Note that under normal conditions axonal injury leads to both LTH-E and LTH-M. Also, both phenomena appear to depend on gene expression, possibly involving CREB activity (Dash et al. 1998), but we do not know the specific genes that are involved in each of these long-term, injury-induced changes. Obviously, the patterns of gene activation that produce LTH-E must differ somewhat from those that produce LTH-M. The dashed lines in A and B represent hypothetical paths for which there is no evidence at present in Aplysia.
Damage to the mammalian nervous system stimulates the release of growth factors, which are important for the responses of neurons to injury, such as sprouting and regeneration (Brown et al. 1991; Wieloch and Nikolich 2006). We found that Aplysia hemolymph, which contains as yet unidentified growth factors (Schacher and Proshansky 1983), was required for the sprouting-like increase in the outgrowth of sensory neurons triggered by axotomy (Fig. 2). However, the long-term hyperexcitability observed in sensory neurons after injury does not appear to require growth factors because it developed and was expressed in the absence of hemolymph (Fig. 1 and Ambron et al. 1996). One significant caveat to these conclusions is that we have no way of knowing the extent to which the washouts we used (methods) actually removed hemolymph-derived factors from the cell cultures. Such factors can bind to the substrate and some of them may have remained behind after washout. Nonetheless, the fact that we observed striking differences between axotomy-induced hyperexcitability and axotomy-induced hypermorphologenesis with respect to the consequences of ostensible hemolymph removal indicates that the washouts must have eliminated at least some critical hemolymph-derived factor(s) from the cultures.
A puzzling result from the present study is that, although growth factors were not necessary for injury-induced LTH-E, the Trk inhibitor K252a blocked the induction of LTH-E (Fig. 3). This result implies that injury can directly activate Trks in Aplysia sensory neurons, independently of growth factors. In support of this idea, ligand-independent activation of TrkA has been observed in nerve growth factor–deprived sympathetic neurons (Wang et al. 2005). A Trk-like receptor has been identified and cloned from the Aplysia nervous system (Ormond et al. 2004). This receptor, ApTrkl, is expressed in Aplysia sensory neurons, but whether ApTrk1 can be directly activated by injury is not known.
Previous evidence indicates that, when the axons of sensory neurons are injured in vivo, PKG is activated at the site of injury; moreover, inhibitors of PKG can block axotomy-induced LTH-E in sensory neurons (Sung et al. 2004). Once activated, PKG stimulates gene transcription via activation of apMAPK (Sung and Ambron 2004), the Aplysia homologue of ERK2 (Michael et al. 1998). Because LTH-E was unaffected by the MEK1/2 inhibitor U0126 (Fig. 6), LTH-E may involve direct activation of apMAPK by PKG (see Sung and Ambron 2004); however, the inhibition of injury-induced LTH-E by the Trk inhibitor K252a (Fig. 3) indicates that LTH-E requires additional signals. Possibly, Trk signaling and the PKG pathway interact in sensory neurons. A potential locus for such an interaction is the cell nucleus. Trk receptors can be retrogradely transported to the nucleus, where they phosphorylate the cyclic AMP response element binding protein (CREB; see following text) (Riccio et al. 1997). LTH-E may therefore involve Trk-dependent phosphorylation of CREB (Fig. 10A). Another potential explanation for the blockade of LTH-E by K252a is an interaction between Trk and ERK/MAPK via PKC. We found that inhibition of PKC by Bis-I blocked LTH-E (Fig. 5). This is consistent with previous evidence that activation of PKC via phorbol ester can produce a long-term enhancement of excitability that resembles the effect of axotomy (Manseau et al. 1998). Furthermore, there is ample precedent for interactions between PKC and ERK/MAPK signaling in the nervous system (e.g., Kawasaki et al. 2004).
The long-term structural changes induced by axotomy in our experiments clearly depend on MEK1/2 activity because they were blocked by U0126 (Fig. 7). The finding that nerve injury activates apMAPK in sensory neurons (Sung and Ambron 2004) provides additional support for the involvement of the ERK/MAPK signaling pathway in injury-induced structural changes in sensory neurons.
We observed that Control sensory neurons can exhibit significant increases in both excitability and neuritic outgrowth. This is apparent in the experiments involving DMSO (Figs. 8 and 9). We attribute the significant cellular changes in Control neurons to signals triggered by axonal injury during the original dissociation of the neurons from the CNS (also see Ambron et al. 1996; Bedi and Glanzman 2001; Bedi et al. 1998). Because the morphological changes in Control neurons were not disrupted by U0126, MEK1/2 activity must not be required for this ongoing outgrowth in sensory neurons, as opposed to the acute outgrowth that occurs within 24 h after axotomy in vitro. This is interesting because it is the first specific molecular distinction we have discovered between the signaling pathways in sensory neurons that mediate the ongoing effects of dissociation-induced axonal injury and those induced by in vitro axotomy.
Results from studies of long-term memory in Aplysia suggest that the structural changes induced in sensory neurons by axotomy are mediated, in part, by CREB-dependent gene transcription. During long-term facilitation of sensorimotor synapses of Aplysia, produced by prolonged treatment with 5-HT, apMAPK translocates to the nucleus of sensory neurons (Martin et al. 1997), where it phosphorylates CREB2, the inhibitory form of CREB (Bartsch et al. 1995). Phosphorylation of CREB2 relieves the repression of CREB1, the transcriptional activator form of CREB, by CREB2 (Bartsch et al. 1998). apMAPK also phosphorylates the CCAAT enhancer-binding protein (C/EBP), a transcription factor and one of the immediate-early genes downstream from CREB1 (Alberini et al. 1994; Michael et al. 1998). Activation of CREB1 during long-term facilitation produces long-term morphological changes in Aplysia sensory neurons, including enhanced neuritic outgrowth (Bartsch et al. 1995; Casadio et al. 1999), that mimic those observed after axotomy. Therefore CREB and C/EBP possibly play critical roles in the structural changes we observed in both Control and Axotomized sensory neurons. In support of this idea Dash et al. (1998) found that the binding of proteins to a CRE sequence-containing probe was increased after injury of the axons of Aplysia motor neurons and that both the number and length of new axon collaterals were enhanced in regenerating motor neurons following injection of a CRE sequence-containing plasmid. In addition to CRE-mediated gene expression, the axotomy-induced outgrowth may have been supported, in part, by utilization of preexisting proteins or, more likely, by local protein synthesis. Weragoda et al. (2004) reported that LTH-E of sensory axons of Aplysia, induced by either injury or local depolarization, requires local protein synthesis, and the same is likely to hold true for LTH-M (see also Schacher and Wu 2002; Villareal et al. 2007).
The stimulation of outgrowth produced by K252a (Fig. 4) in the present study is intriguing. The structural change in unaxotomized Control neurons treated with K252a was greater than that typically observed in Axotomized neurons in the absence of this drug (compare Bedi and Glanzman 2001 and Fig. 9 in Bedi et al. 1998). Axotomy plus K252a did not produce significantly greater outgrowth than that in the Control neurons treated with the drug. Therefore K252a appeared to occlude the effect of acute axonal injury, which indicates that the drug and axotomy both affect processes that regulate growth. How might K252a stimulate neurite outgrowth in sensory neurons? Hislop et al. (2004) identified a novel Trk (nork) in Aplysia that inhibits the outgrowth of Aplysia sensory neurons in culture when it is overexpressed in these neurons. Hislop et al. suggest that nork is normally involved in inhibiting neurite outgrowth in Aplysia. Possibly, the dramatic stimulatory action of K252a on sensory neuron outgrowth in our experiments was due to inhibition of nork.
We occasionally observed a disjunction between the results from our two measures of morphological change: axonal branch point number and axonal length. For example, K252a produced a highly significant increase in the number of axonal branch points in both Control and Axotomized sensory neurons (Fig. 4); however, there was no significant change in axonal length for the Control neurons and a significant decrease in axonal length for the Axotomized neurons (Table 2). Similarly, whereas there was a significantly greater increase in the number of axonal branch points in Axotomized than in Control neurons in the presence of Bis-I, there was no apparent difference between Axotomized and Control neurons with respect to the change in axonal length (Table 3). Smith and Skene (1997) showed that dorsal root ganglion (DRG) neurons in culture exhibit two different types of growth, which they refer to as “elongating” and “arborizing.” These two patterns of growth in DRG neurons depend on different patterns of gene transcription. Our morphological results are consistent with the idea that axotomy and pharmacological inhibitors (e.g., K252a) may differentially affect arborizing and elongating patterns of growth in cultured Aplysia sensory neurons.
TABLE 3.
Additional data from experiments involving Bis-I
| Bisindolymaleimide I | ||||||
|---|---|---|---|---|---|---|
| Parameter | Control |
Axotomized | ||||
| Day 1 | Day 2 | P | Day 1 | Day 2 | P | |
| Spike threshold, nA | 0.5 ± 0.04 | 0.5 ± 0.06 | NS | 0.5 ± 0.03 | 0.4 ± 0.03 | <0.03 |
| Membrane potential, mV | 48.2 ± 1.7 | 48.5 ± 2.0 | NS | 50.0 ± 1.1 | 49.1 ± 1.1 | NS |
| Membrane resistance, MΩ | 109.0 ± 5.3 | 100.0 ± 10.0 | NS | 103.0 ± 8.9 | 112.0 ± 6.2 | NS |
| Axonal length, μm | 284.0 ± 25.0 | 392.0 ± 40.0 | <0.004 | 272.0 ± 22.0 | 384.0 ± 38.0 | <0.002 |
There was a significant decrease in spike threshold for Axotomized, but not Control, neurons. There were no significant changes in either membrane potential or membrane resistance in the experiments. Both Control and Axotomized neurons exhibited significant increases in axonal length.
In summary, we have delineated distinct cellular pathways that regulate long-term electrophysiological and morphological changes in Aplysia sensory neurons following axotomy (Fig. 10). Further elucidation of these pathways is likely to lead to general insights into the mechanisms of axonal regeneration. Such insights, in turn, may assist in the development of clinical treatments for injuries of the CNS, particularly spinal cord injury.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants R01 NS-29563 to D. L. Glanzman and T32 NS-07449 to S. S. Bedi.
TABLE 4.
Additional data from experiments that used U0126
| U0126 | ||||||
|---|---|---|---|---|---|---|
| Parameter | Control |
Axotomized | ||||
| Day 1 | Day 2 | P | Day 1 | Day 2 | P | |
| Spike threshold, nA | 0.6 ± 0.02 | 0.5 ± 0.04 | <0.05 | 0.54 ± 0.03 | 0.45 ± 0.03 | <0.04 |
| Membrane potential, mV | 45.2 ± 0.9 | 43.6 ± 1.1 | NS | 44.4 ± 0.7 | 42.1 ± 0.8 | NS |
| Membrane resistance, MΩ | 66.4 ± 5.5 | 63.4 ± 3.3 | NS | 66.9 ± 4.2 | 71.0 ± 3.7 | NS |
| Axonal length, μm | 714.0 ± 52.0 | 915.0 ± 82.0 | <0.002 | 768.0 ± 60.0 | 820.0 ± 59.0 | NS |
There were significant decreases in spike threshold in both Control and Axotomized neurons. We have occasionally observed a modest, albeit significant, long-term increase in the excitability of Control neurons in our previous studies (Bedi and Glanzman 2001, and results; Bedi et al. 1998). No other significant electrophysiological changes were observed in the present experiments with U0126. There was also a significant increase in axonal length in the Control, but not in the Axotomized, sensory neurons.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Alberini et al. 1994.Alberini CM, Ghirardi M, Metz R, Kandel ER. C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia. Cell 76: 1099–1114, 1994. [DOI] [PubMed] [Google Scholar]
- Ambron et al. 1996.Ambron RT, Zhang XP, Gunstream JD, Povelones M, Walters ET. Intrinsic injury signals enhance growth, survival, and excitability of Aplysia neurons. J Neurosci 16: 7469–7477, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeles et al. 1998.Angeles TS, Yang SX, Steffler C, Dionne CA. Kinetics of trkA tyrosine kinase activity and inhibition by K-252a. Arch Biochem Biophys 349: 267–274, 1998. [DOI] [PubMed] [Google Scholar]
- Bartsch et al. 1998.Bartsch D, Casadio A, Karl KA, Serodio P, Kandel ER. CREB1 encodes a nuclear activator, a repressor, and a cytoplasmic modulator that form a regulatory unit critical for long-term facilitation. Cell 95: 211–223, 1998. [DOI] [PubMed] [Google Scholar]
- Bartsch et al. 1995.Bartsch D, Ghirardi M, Skehel PA, Karl KA, Herder SP, Chen M, Bailey CH, Kandel ER. Aplysia CREB2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural change. Cell 83: 979–992, 1995. [DOI] [PubMed] [Google Scholar]
- Bedi and Glanzman 2001.Bedi SS, Glanzman DL. Axonal rejoining inhibits injury-induced long-term changes in Aplysia sensory neurons in vitro. J Neurosci 21: 9667–9677, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi and Glanzman 2002.Bedi SS, Glanzman DL. Inhibitor of MEK1/2 inhibits hypermorphogenesis of Aplysia sensory neurons in vitro following axotomy. Soc Neurosci Abstr 334.12, 2002. [Google Scholar]
- Bedi et al. 1998.Bedi SS, Salim A, Chen S, Glanzman DL. Long-term effects of axotomy on excitability and growth of isolated Aplysia sensory neurons in cell culture: potential role of cAMP. J Neurophysiol 79: 1371–1383, 1998. [DOI] [PubMed] [Google Scholar]
- Berg et al. 1992.Berg MM, Sternberg DW, Parada LF, Chao MV. K-252a inhibits nerve growth factor-induced trk proto-oncogene tyrosine phosphorylation and kinase activity. J Biol Chem 267: 13–16, 1992. [PubMed] [Google Scholar]
- Bittner 1991.Bittner GD Long-term survival of anucleate neurons and its implications for nerve regeneration. Trends Neurosci 14: 188–193, 1991. [DOI] [PubMed] [Google Scholar]
- Brown et al. 1991.Brown MC, Perry VH, Lunn ER, Gordon S, Heumann R. Macrophage dependence of peripheral sensory nerve regeneration: possible involvement of nerve growth factor. Neuron 6: 359–370, 1991. [DOI] [PubMed] [Google Scholar]
- Casadio et al. 1999.Casadio A, Martin KC, Giustetto M, Zhu H, Chen M, Bartsch D, Bailey CH, Kandel ER. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell 99: 221–237, 1999. [DOI] [PubMed] [Google Scholar]
- Chao 2003.Chao MV Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci 4: 299–309, 2003. [DOI] [PubMed] [Google Scholar]
- Dash et al. 1998.Dash PK, Tian LM, Moore AN. Sequestration of cAMP response element-binding proteins by transcription factor decoys causes collateral elaboration of regenerating Aplysia motor neuron axons. Proc Natl Acad Sci USA 95: 8339–8344, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies et al. 2000.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351: 95–105, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanzman et al. 1989.Glanzman DL, Kandel ER, Schacher S. Identified target motor neuron regulates neurite outgrowth and synapse formation of Aplysia sensory neurons in vitro. Neuron 3: 441–450, 1989. [DOI] [PubMed] [Google Scholar]
- Goldberg and Barres 2000.Goldberg JL, Barres BA. The relationship between neuronal survival and regeneration. Annu Rev Neurosci 23: 579–612, 2000. [DOI] [PubMed] [Google Scholar]
- Gunstream et al. 1995.Gunstream JD, Castro GA, Walters ET. Retrograde transport of plasticity signals in Aplysia sensory neurons following axonal injury. J Neurosci 15: 439–448, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hislop et al. 2004.Hislop J, Dyer JR, Scott D, van Kesteren RE, Sossin WS. Characterization of a novel molluskan tyrosine kinase receptor that inhibits neurite regeneration. J Neurobiol 60: 127–136, 2004. [DOI] [PubMed] [Google Scholar]
- Hu et al. 2007.Hu JY, Chen Y, Schacher S. Multifunctional role of protein kinase C in regulating the formation and maturation of specific synapses. J Neurosci 27: 11712–11724, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir et al. 2001.Kabir N, Schaefer AW, Nakhost A, Sossin WS, Forscher P. Protein kinase C activation promotes microtubule advance in neuronal growth cones by increasing average microtubule growth lifetimes. J Cell Biol 152: 1033–1044, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kase et al. 1987.Kase H, Iwahashi K, Nakanishi S, Matsuda Y, Yamada K, Takahashi M, Murakata C, Sato A, Kaneko M. K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem Biophys Res Commun 142: 436–440, 1987. [DOI] [PubMed] [Google Scholar]
- Kawasaki et al. 2004.Kawasaki Y, Kohno T, Zhuang ZY, Brenner GJ, Wang H, Van Der Meer C, Befort K, Woolf CJ, Ji RR. Ionotropic and metabotropic receptors, protein kinase A, protein kinase C, and Src contribute to C-fiber-induced ERK activation and cAMP response element-binding protein phosphorylation in dorsal horn neurons, leading to central sensitization. J Neurosci 24: 8310–8321, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin and Walters 1999.Lewin MR, Walters ET. Cyclic GMP pathway is critical for inducing long-term sensitization of nociceptive sensory neurons. Nat Neurosci 2: 18–23, 1999. [DOI] [PubMed] [Google Scholar]
- Liao et al. 1999.Liao X, Gunstream JD, Lewin MR, Ambron RT, Walters ET. Activation of protein kinase A contributes to the expression but not the induction of long-term hyperexcitability caused by axotomy of Aplysia sensory neurons. J Neurosci 19: 1247–1256, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manseau et al. 1998.Manseau R, Sossin WS, Castellucci VF. Long-term changes in excitability induced by protein kinase C activation in Aplysia sensory neurons. J Neurophysiol 79: 1210–1218, 1998. [DOI] [PubMed] [Google Scholar]
- Martin et al. 1997.Martin KC, Michael D, Rose JC, Barad M, Casadio A, Zhu H, Kandel ER. MAP kinase translocates into the nucleus of the presynaptic cell and is required for long-term facilitation in Aplysia. Neuron 18: 899–912, 1997. [DOI] [PubMed] [Google Scholar]
- Michael et al. 1998.Michael D, Martin KC, Seger R, Ning MM, Baston R, Kandel ER. Repeated pulses of serotonin required for long-term facilitation activate mitogen-activated protein kinase in sensory neurons of Aplysia. Proc Natl Acad Sci USA 95: 1864–1869, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormond et al. 2004.Ormond J, Hislop J, Zhao Y, Webb N, Vaillaincourt F, Dyer JR, Ferraro G, Barker P, Martin KC, Sossin WS. ApTrkl, a Trk-like receptor, mediates serotonin- dependent ERK activation and long-term facilitation in Aplysia sensory neurons. Neuron 44: 715–728, 2004. [DOI] [PubMed] [Google Scholar]
- Purcell and Carew 2001.Purcell AL, Carew TJ. Modulation of excitability in Aplysia tail sensory neurons by tyrosine kinases. J Neurophysiol 85: 2398–2411, 2001. [DOI] [PubMed] [Google Scholar]
- Raff et al. 2002.Raff MC, Whitmore AV, Finn JT. Axonal self-destruction and neurodegeneration. Science 296: 868–871, 2002. [DOI] [PubMed] [Google Scholar]
- Riccio et al. 1997.Riccio A, Pierchala BA, Ciarallo CL, Ginty DD. An NGF-TrkA-mediated retrograde signal to transcription factor CREB in sympathetic neurons. Science 277: 1097–1100, 1997. [DOI] [PubMed] [Google Scholar]
- Schacher and Proshansky 1983.Schacher S, Proshansky E. Neurite regeneration by Aplysia neurons in dissociated cell culture: modulation by Aplysia hemolymph and the presence of the initial segment. J Neurosci 3: 2403–2034, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacher and Wu 2002.Schacher S, Wu F. Synapse formation in the absence of cell bodies requires protein synthesis. J Neurosci 22: 1831–1839, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab 2002.Schwab ME Repairing the injured spinal cord. Science 295: 1029–1031, 2002. [DOI] [PubMed] [Google Scholar]
- Segal and Greenberg 1996.Segal RA, Greenberg ME. Intracellular signaling pathways activated by neurotrophic factors. Annu Rev Neurosci 19: 463–489, 1996. [DOI] [PubMed] [Google Scholar]
- Smith and Skene 1997.Smith DS, Skene JH. A transcription-dependent switch controls competence of adult neurons for distinct modes of axon growth. J Neurosci 17: 646–658, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossin 2007.Sossin WS Isoform specificity of protein kinase Cs in synaptic plasticity. Learn Mem 14: 236–246, 2007. [DOI] [PubMed] [Google Scholar]
- Steffensen et al. 1995.Steffensen I, Dulin MF, Walters ET, Morris CE. Peripheral regeneration and central sprouting of sensory neuron axons in Aplysia californica following nerve injury. J Exp Biol 198: 2067–2078, 1995. [DOI] [PubMed] [Google Scholar]
- Sung and Ambron 2004.Sung YJ, Ambron RT. Pathways that elicit long-term changes in gene expression in nociceptive neurons following nerve injury: contributions to neuropathic pain. Neurol Res 26: 195–203, 2004. [DOI] [PubMed] [Google Scholar]
- Sung et al. 2001.Sung YJ, Povelones M, Ambron RT. RISK-1: a novel MAPK homologue in axoplasm that is activated and retrogradely transported after nerve injury. J Neurobiol 47: 67–79, 2001. [DOI] [PubMed] [Google Scholar]
- Sung et al. 2004.Sung YJ, Walters ET, Ambron RT. A neuronal isoform of protein kinase G couples mitogen-activated protein kinase nuclear import to axotomy-induced long-term hyperexcitability in Aplysia sensory neurons. J Neurosci 24: 7583–7595, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton et al. 2004.Sutton MA, Bagnall MW, Sharma SK, Shobe J, Carew TJ. Intermediate-term memory for site-specific sensitization in Aplysia is maintained by persistent activation of protein kinase C. J Neurosci 24: 3600–3609, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt 2004.Sweatt JD Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol 14: 311–317, 2004. [DOI] [PubMed] [Google Scholar]
- Toullec et al. 1991.Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, and et al. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem 266: 15771–15781, 1991. [PubMed] [Google Scholar]
- Ungless et al. 2002.Ungless MA, Gasull X, Walters ET. Long-term alteration of S-type potassium current and passive membrane properties in Aplysia sensory neurons following axotomy. J Neurophysiol 87: 2408–2420, 2002. [DOI] [PubMed] [Google Scholar]
- Villareal et al. 2007.Villareal G, Li Q, Cai D, Glanzman DL. The role of rapid, local postsynaptic protein synthesis in learning-related synaptic facilitation in Aplysia. Curr Biol 17: 2073–2080, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters et al. 1991.Walters ET, Alizadeh H, Castro GA. Similar neuronal alterations induced by axonal injury and learning in Aplysia. Science 253: 797–799, 1991. [DOI] [PubMed] [Google Scholar]
- Wang et al. 2005.Wang LH, Paden AJ, Johnson EM Jr. Mixed-lineage kinase inhibitors require the activation of Trk receptors to maintain long-term neuronal trophism and survival. J Pharmacol Exp Ther 312: 1007–1019, 2005. [DOI] [PubMed] [Google Scholar]
- Watson et al. 2001.Watson FL, Heerssen HM, Bhattacharyya A, Klesse L, Lin MZ, Segal RA. Neurotrophins use the Erk5 pathway to mediate a retrograde survival response. Nat Neurosci 4: 981–988, 2001. [DOI] [PubMed] [Google Scholar]
- Weragoda et al. 2004.Weragoda RM, Ferrer E, Walters ET. Memory-like alterations in Aplysia axons after nerve injury or localized depolarization. J Neurosci 24: 10393–10401, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weragoda and Walters 2007.Weragoda RM, Walters ET. Serotonin induces memory-like, rapamycin-sensitive hyperexcitability in sensory axons of Aplysia that contributes to injury responses. J Neurophysiol 98: 1231–1239, 2007. [DOI] [PubMed] [Google Scholar]
- Wieloch and Nikolich 2006.Wieloch T, Nikolich K. Mechanisms of neural plasticity following brain injury. Curr Opin Neurobiol 16: 258–264, 2006. [DOI] [PubMed] [Google Scholar]
- Zhao et al. 2007.Zhao P, Waxman SG, Hains BC. Extracellular signal-regulated kinase-regulated microglia-neuron signaling by prostaglandin E2 contributes to pain after spinal cord injury. J Neurosci 27: 2357–2368, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu et al. 1994.Zhu H, Wu F, Schacher S. Aplysia cell adhesion molecules and serotonin regulate sensory cell- motor cell interactions during early stages of synapse formation in vitro. J Neurosci 14: 6886–6900, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]