Table 1.
Physical data of 2,3-diaryl-1,3-thiazolidin-4-one derivatives.
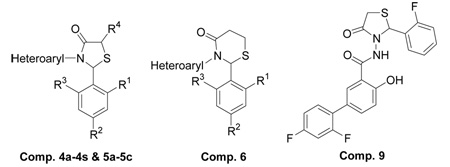 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Comp. | Heteroaryl | R1 | R2 | R3 | R4 | MWt | % yield | m.p. °C |
| 4a | Pyridin-2-yl | Quinolin-4-yl | H | 307 | 75 | - | ||
| 4b | Pyridin-2-yl | H | NMe2 | H | H | 299 | 68 | - |
| 4c | Pyridin-2-yl | H | F | H | H | 274 | 78 | - |
| 4d | 6-methyl-pyridin-2-yl | Pyridin-3-yl | H | 271 | 55 | 104–106 | ||
| 4e | 6-methyl-pyridin-2-yl | H | NMe2 | H | H | 313 | 72 | 133–135 |
| 4f | Pyridin-3-ylmethyl | Cl | H | Cl | H | 339 | 92 | - |
| 4g | 4-methyl-6-trifluoromethyl-pyrimidin-2-yl | Cl | H | Cl | H | 408 | 50 | 111–115 |
| 4h | 4-methyl-6-trifluoromethyl-pyrimidin-2-yl | Cl | H | F | H | 392 | 47 | 92–94 |
| 4i | 4-methyl-6-trifluoromethyl-pyrimidin-2-yl | Br | H | Br | H | 497 | 36 | 133–137 |
| 4j | 4-methyl-6-phenyl-pyrimidin-2-yl | Cl | H | Cl | H | 416 | 48 | 168–170 |
| 4k | 4-methyl-6-phenyl-pyrimidin-2-yl | Cl | H | F | H | 400 | 41 | 138–140 |
| 4l | 4-phenyl-6-trifluoromethyl-pyrimidin-2-yl | Cl | H | Cl | H | 470 | 46 | 206–208 |
| 4m | 4,6-diphenyl-pyrimidin-2-yl | Cl | H | Cl | H | 478 | 38 | 206–208 |
| 4n | 4,6-diphenyl-pyrimidin-2-yl | Cl | H | F | H | 462 | 30 | 176–178 |
| 4o | 4,6-diphenyl-pyrimidin-2-yl | F | H | F | H | 445 | 28 | 192–194 |
| 4p | Quinolin-2-yl | Cl | H | F | H | 358 | 56 | 144–146 |
| 4q | Furan-2-ylmethyl | Cl | H | Cl | H | 328 | 92 | 96–98 |
| 4r | Thiophen-2-ylmethyl | Br | H | Br | H | 433 | 64 | - |
| 4s | 5-ethyl-[1,3,4]-thiadiazol-2-yl | F | H | F | H | 327 | 44 | 110–114 |
| 5a | Pyridin-2-yl | Cl | H | Cl | CH3 | 339 | 80 | 85–89 |
| 5b | Pyridin-3-ylmethyl | Cl | H | Cl | CH3 | 353 | 90 | - |
| 5c | Furan-2-ylmethyl | Cl | H | Cl | CH3 | 342 | 88 | - |
| 6 | Furan-2-ylmethyl | Cl | H | Cl | H | 342 | 90 | - |
| 7 | Pyridin-2-yl | 1-benzyl-piperidinyl | H | 339 | 50 | - | ||
| 8a | Furan-2-ylmethyl | Cl | H | Cl | H | 344 | 78 | 155–158 |
| 9 | 2',4'-Difluoro-4-hydroxy-biphenyl-3-carboxylic acid [2-(2-fluoro-phenyl)-4-oxo-thiazolidin-3-yl]-amide | - | - | - | ||||
sulfoxide.
