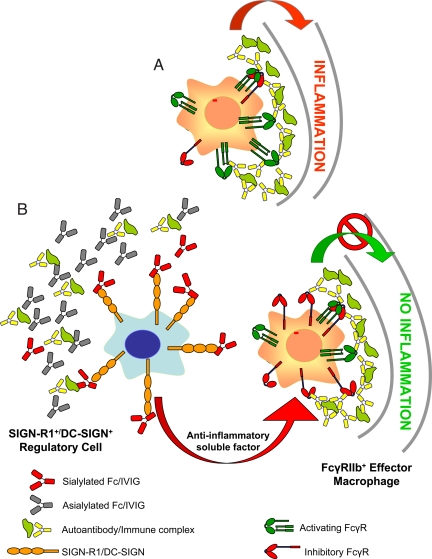
Fig. 7.
A model for the anti-inflammatory activity of 2,6-sialylated Fc. Features of this model have been presented previously. (A) Under inflammatory and autoimmune conditions, immune complexes formed by autoantigens and self-reactive antibodies bind activating FcR expressed by inflammatory macrophages, leading to their activation. (B) 2,6-Sialylated Fc, found in preparations of i.v. gamma globulin (IVIG), engages a lectin on the surface of a regulatory macrophage population in the spleen found in the marginal zone, now identified as SIGN-R1. Engagement of this lectin induces a cellular program that results in the secretion of anti-inflammatory, soluble mediators that target effector macrophages found at the site of tissue inflammation where pathogenic immune complexes are deposited. These effector macrophages respond to the anti-inflammatory mediators by increasing surface expression of the inhibitory FcgRIIB receptor, thereby altering the threshold concentration of immune complexes necessary to trigger macrophage activation and subsequent inflammation. The net result of this pathway then is to attenuate autoantibody mediated inflammation and tissue pathology. The homologous pathway in the human differs in that the lectin is DC-SIGN and the regulatory cells are dendritic cells and thus, not restricted to the spleen.