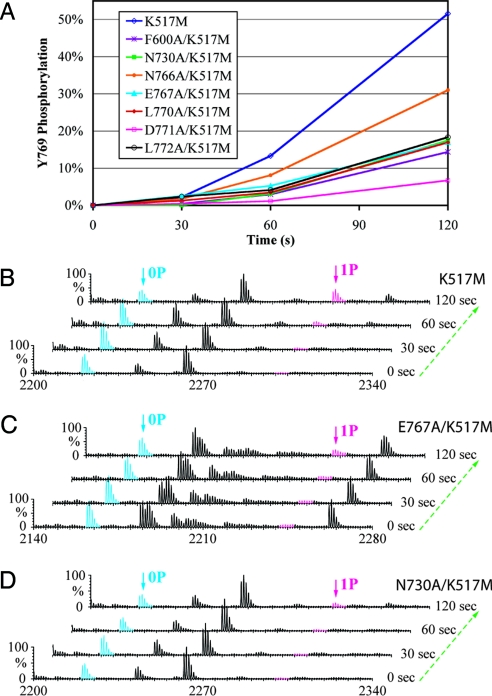
Fig. 3.
Comparison of the time course of trans-phosphorylation on Y769 in WT and mutant kinase substrates by time-resolved MALDI Q-TOF MS. (A) Relative to the parent WT kinase substrate (K517M), trans-phosphorylation on Y769 is reduced in the 7 mutant kinase substrates harboring structure-based mutations. Ion counts of the phosphopeptide and its unphosphorylated version are used to estimate the percentage of phosphorylation of Y769. (B–D) Time-resolved MALDI Q-TOF MS spectra show the progression of the trans-phosphorylation reaction on Y769 peptide in K517M (B), E767A/K517M (C), and N730/K517M (D) mutant substrates. 0P and 1P denote the FGFR2K peptide in unphosphorylated and single-phosphorylated states, respectively.