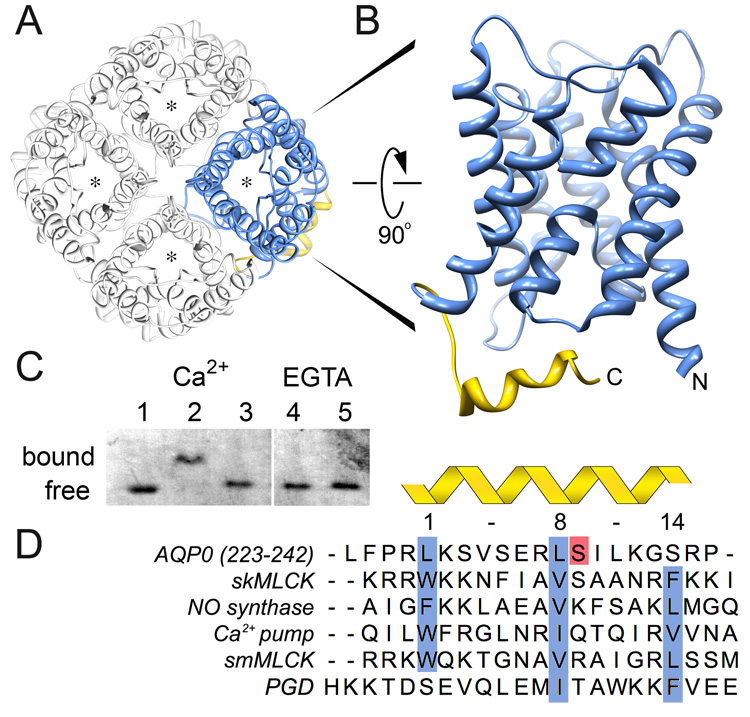
Figure 2. Identification of the AQP0-Calmodulin binding domain and effect of serine phosphorylation.
A, Aquaporins form tetrames in which each monomer forms a functional and independent water pore (asterisk) (Shi et al., 1994). The AQP0 tetramer is viewed from the extracellular side of the cell membrane (Protein Data Bank Accession number 2B6O). B, Side view of an AQP0 monomer highlighting the cytoplasmic C-terminal α-helix as the calmodulin binding domain (AQP0CBD, yellow). C, Native gel electrophoresis of calmodulin bound to AQP0CBD. A clear shift in the calmodulin band is seen in the presence of Ca2+ (Lanes 1 versus 2) but not when Ser 235 is phosphorylated (Lane 3) or in the presence of EGTA even with excess peptide (Lanes 4 and 5, respectively). D, Sequence alignment of AQP0CBD and 4 other 1-8-14 calmodulin binding motifs (highlighted in blue) from different proteins as indicated in the figure. The AQP0CBD is missing the large hydrophobic residue at position 14. Ser 235 is highlighted in red and is the site of phosphorylation assayed in this study. The sequence of the non-cononical plant glutamate decarboxylase (PGD) CaM binding site is at the bottom.