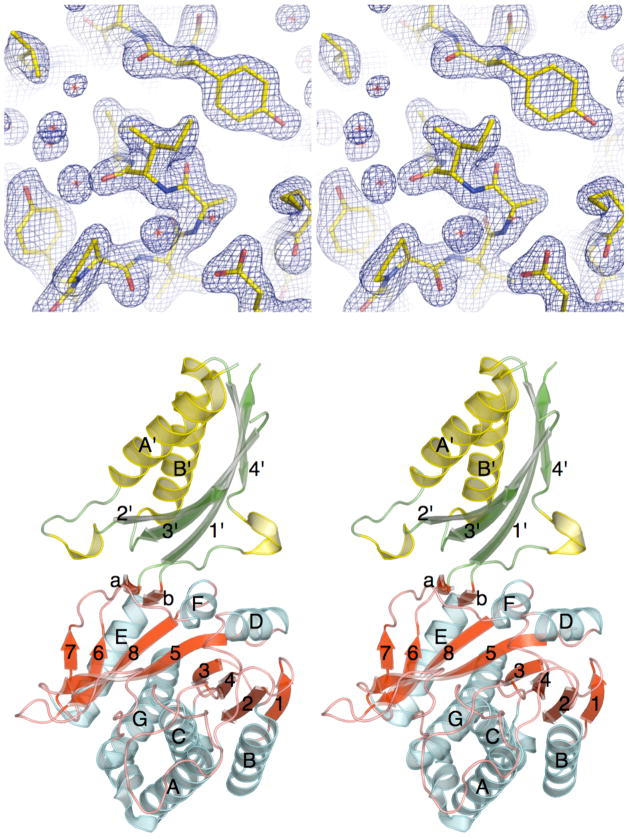
Figure 1.
The X-ray crystal structure of ILL2. (A) Stereo view of a 2mFo−DFc electron density map of the refined ILL2 model around Ile92, which adopts an unusual backbone conformation. The map is contoured at 1.3σ level. (B) Ribbon diagram of ILL2 in stereo representation shows that this enzyme consists of two distinct domains. Strands and helices in both the catalytic domain (bottom, red and cyan) and the satellite domain (top, green and yellow) are numbered in order of their appearance in respective domains. The catalytic domain of ILL2 consists of the central 8-stranded β-sheet (red) sandwiched between five and two helices on either side (cyan). The satellite domain (top) is inserted between β-strand 6 and helix E of the catalytic domain.