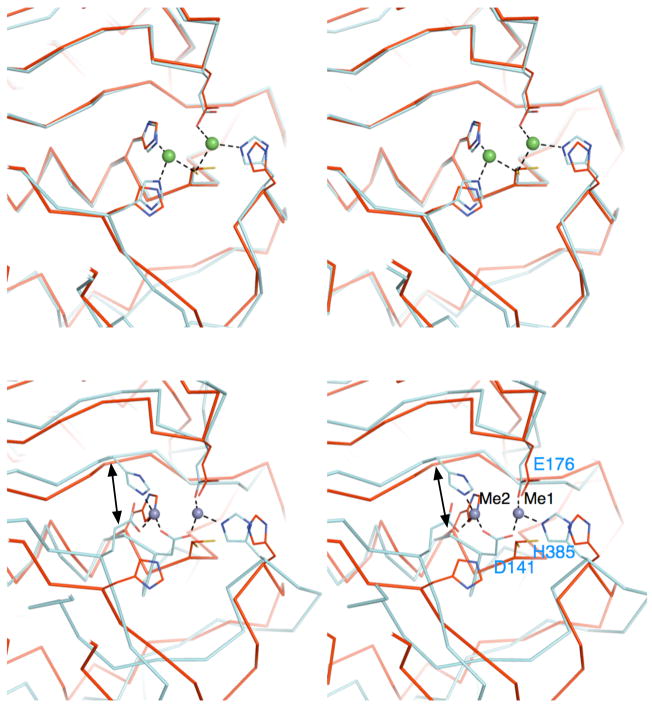
Figure 2.
Comparison of the active sites of ILL2 and its structural homologs. (A) A stereo view the ribbon trace of superimposed ILL2 (red; PDB ID 1xmb) and yxeP from B. subtilis (cyan; PDB ID 1ysj). Catalytic domains of ILL2 and yxeP are highly similar and superimpose with an all-atom rmsd of 0.5 Å. The residues involved in coordination of Metal 1 and Metal 2 (green spheres) in yxeP are shown as cyan sticks. The analogous residues present in the apo-ILL2 structure are also shown (red sticks) and annotated by labels. (B) A stereo view of the ribbon trace of superimposed ILL2 (red) and CPG2 (cyan, PDB ID 1cg2). The figure is given in the same orientation as in Figure 2a to allow for easy comparison of all structures. The residues involved in metal coordination are shown in cyan sticks and are annotated for CPG2. Asp141 of CPG2 serves as a bidentate ligand in metal coordination, replacing Cys137 of ILL2. The double arrow points to the functionally related metal ligand that originates from topologically distinct positions in ILL2 (His139) and CPG2 (His112).