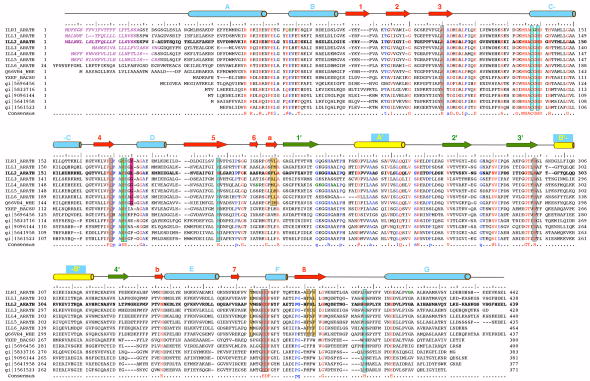
Figure 3.
The multiple sequence alignment of selected ILL2 homologs. The multiple sequence alignment of all known IAA-amino acid hydrolases from Arabidopsis thaliana (first seven entries; UniProt codes ILR1_ARATH through ILL6_ARATH), IAR3 homolog from wheat (UniProt code Q66VR4_WHEAT), and several bacterial ILL2 homologs including yxeP protein from Bacillus subtilis, N-acyl-L-amino acid amidohydrolase from Bacillus clausii (gi|56964568), amino acid amidohydrolase from Lactobacillus acidophilus (gi|58337160), N-acetyldiaminopimelate deacetylase from Lactobacillus salivarius (gi|90961445), hippurate hydrolase from Geobacillus kaustophilus (gi|56419585) and hippurate hydrolase from Bacillus halodurans (gi|15615231). The ILL2 sequence is shown in bold letters and accompanied by a ruler indicating every 5th (:) and 10th (|) residue. Invariant residues of all the aligned sequences are highlighted in red, other highly conserved residues are colored in blue. The important active site residues are boxed and color-coded as in Figure 4a with metal-coordinating residues in cyan, hydrophobic residues that bind the indole ring of a substrate in orange, a putative general acid-base residue in green, a putative amino acid side chain selectivity filter residue in magenta, and other conserved residues in the vicinity of the active site in gray. Secondary structural elements of ILL2 are shown as cylinders (helices) and arrows (β-strands). The numbering and color-coding scheme of the secondary structural elements corresponds to that in Figure 1b. Purple italics letters highlight the predicted signal peptide sequences of A. thaliana amidohydrolases. Bold green letters highlight residues mutated in loss-of-function mutants of A. thaliana ILR1 and IAR3.8,9 Further information about these mutations is given in Table II.