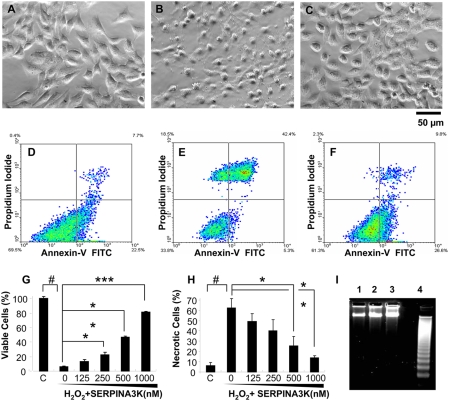
Figure 2. Protective effects of SERPINA3K on rMC-1 cells against H2O2-induced necrosis.
Müller-derived rMC-1 cells were pre-treated with SERPINA3K or BSA for 1 h and then exposed to 400 µM H2O2 for 8 h (A–C, G) and 4 h (D–F, H, I). The protein concentration in the media was brought to 1 µM in each well by addition of BSA. (A–C) Representative phase contrast images showing cell morphology in untreated control (A), cells treated with 1 µM BSA and H2O2 (B), and cells treated with 1 µM SERPINA3K and H2O2 (C). Scale bar, 50 µm. (G) Viable cells were quantified using the MTT assay (mean±SEM, n = 3). (D–F, H) The viable and necrotic cells were analyzed by flow cytometry following staining with PI and Annexin-V; (D) Untreated control; (E) treated with 1 µM BSA and H2O2; (F) treated with 1 µM SERPINA3K and H2O2; (H) quantification of necrotic cells as percentages of total cells (mean±SEM, n = 3). (I) DNA laddering analysis showed no apparent DNA fragmentation in the H2O2-treated cells. Lane 1, control cells; 2, cells treated with H2O2 for 4 h; 3, cells treated with H2O2 and SERPINA3K, and 4, over-grown cells as positive control. # P<0.001, H2O2 treated cells versus control cells. * P<0.05, ** P<0.01, *** P<0.001, the cells treated by different doses of SERPINA3K versus the cells treated only by BSA.