Figure 4.
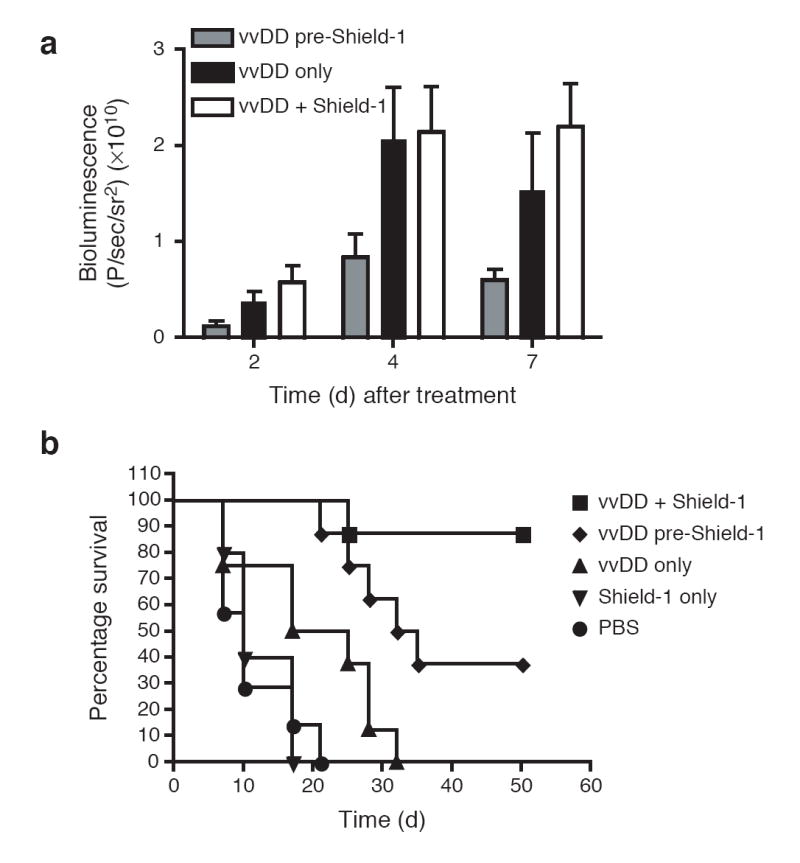
Antitumor benefit of conditional regulation of a targeted gene therapy vector. CD1 nu-/nu- mice bearing subcutaneous HCT116 tumors (150-250 mm3) were treated via a single tail vein injection with either PBS (circles) or the vaccinia strain vvDD expressing both luciferase as well as the L-L106P-TNF-α fusion protein (1 × 108 PFU/mouse). Treated mice also received Shield-1 (10 mg/kg) every 48 hr by three different protocols, starting (i) 24 hr prior to vvDD therapy (diamonds); (ii) 72 hr post-vvDD therapy (squares) or (iii) not at all (triangles) (n=8 mice/group). Tumor-bearing mice that did not receive vvDD therapy were also treated with Shield-1 (10 mg/kg) as a negative control (inverted triangles, n=5). (a) Viral load within the tumor was assayed by measuring constitutive viral gene expression (bioluminescence) for each group at the indicated time points after vvDD treatment. Shield-1 treatment starting 72 hr after vvDD (vvDD + Shield-1) resulted in significantly greater levels of viral gene expression in the tumor than when Shield-1 treatment was started prior to vvDD therapy (vvDD Pre-Shield-1; i.e., constitutive TNF-α expression) (p=0.035 at 2 days; p=0.035 at 4 days and p=0.002 at 7 days). (b) Tumor volume was determined by caliper measurement and mice were sacrificed once tumor volume reached 1.44 cm3. Kaplan-Meier survival graphs are shown, and all surviving mice at 50 days had no detectable tumor. Mice treated with Shield-1 prior to vvDD therapy (i.e., constitutive TNF-α expression) produced significantly enhanced survival relative to mice treated with vvDD alone (p=0.017). Addition of Shield-1 72 hr post-vvDD (vvDD + Shield-1) produced a further significant increase in survival relative to mice treated with Shield-1 prior to vvDD (vvDD pre-Shield-1) (p=0.031).
