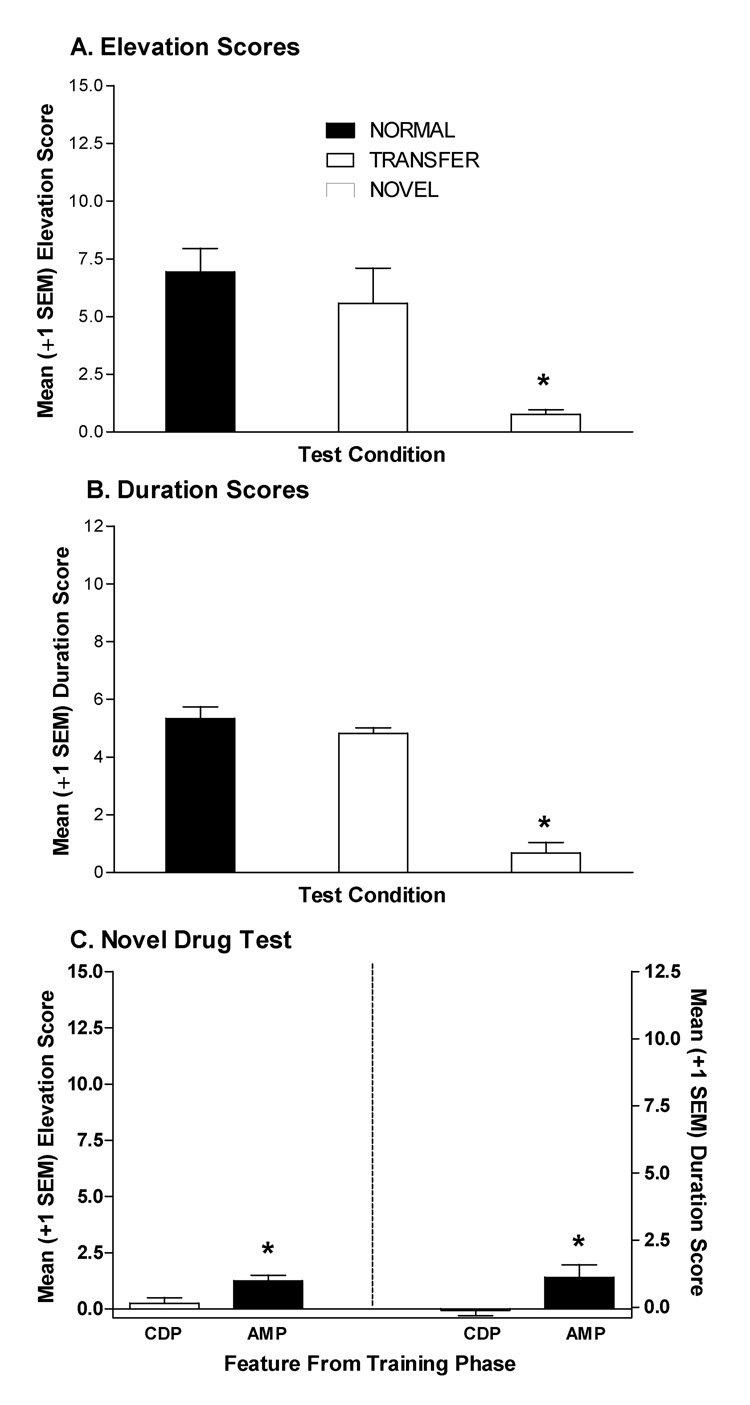
Figure 4.
Mean (±1 SEM) elevation scores (A) and duration scores (B) for the normal and transfer feature-target compound tests and the novel drug feature test for rats in the AMP/CDP group of Experiment 3A. For the “normal” test, rats received injections of AMP or CDP 15 min before a single exposure to the CS normally followed by sucrose in that drug state. For the “transfer” test, rats received injections of AMP or CDP 15 min before a single exposure to the CS followed by sucrose in the other drug state during training. For the novel feature test rats were injected with nicotine (0.1 mg/kg SC) 15 min before a single exposure to one CS from the training phase. The CS presented on the novel drug test was counterbalanced according to modality (light vs. noise) and previous training drug state (AMP vs. CDP). (C) Illustrates mean (±1 SEM) elevation and duration scores from the novel drug test for rats receiving the CS previously presented in the AMP vs. CDP drug state on the novel drug test. All tests were conducted in extinction. * indicates elevation or duration scores differ significantly from normal test condition, p<0.05.