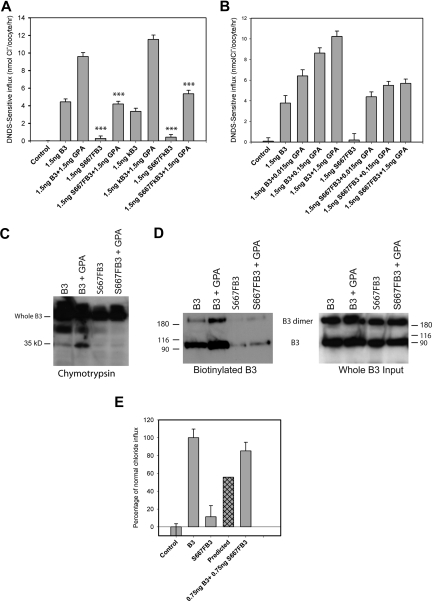
Figure 4.
Chloride influx and surface assays in Xenopus oocytes. Band 3 cRNA (B3), kidney band 3 cRNA (kB3), S667F band 3 cRNA (S667F-B3), or S667F kidney band 3 cRNA (S667F-kB3) was injected into Xenopus oocytes either alone or coinjected with GPA or band 3 at concentrations indicated. DNDS-sensitive chloride influx (1 hour) was measured 24 hours after injection with the cRNA using groups of 12 to 15 oocytes. Results are shown as means plus or minus SEM. Significance level is for comparisons of influx between B3 and S667F-B3, or B3 plus GPA and S667F-B3 plus GPA, or kB3 and S667F-kB3, or kB3 plus GPA and S667F-kB3 plus GPA (sample by Student t test; ***P < .001). For the chymotrypsin assay and the biotinylation assay, oocytes were injected with 5 ng of B3 or S667F-B3 and 1.5 ng of GPA and allowed to express protein for 24 hours. The oocytes were then subjected to a chymotrypsin assay or biotinylated as outlined in “Cell-surface protease assay and biotinylation of oocytes.” (A) S667F-B3 has very little chloride influx when expressed alone in oocytes, and this is incompletely rescued by coexpression of GPA in both S667F-B3 and S667F-kB3 to approximately 50% of wild-type B3 + GPA or kB3 + GPA. (B) Comparison of the effects of coexpression of 0.015 to 1.5 ng GPA cRNA on normal B3 and S667F-B3 chloride influx. Although GPA dose-dependently increased wild-type chloride influx, the enhancement effect of GPA was observed to be maximal at 0.15 ng with S667F-B3 (representative of 3 independent experiments) and was saturated. (C,D) Representative chymotrypsin and biotinylation blots after 24 hours' expression using anti–band 3 C-terminal antibody BRIC155 to detect B3. The chymotrypsin gel was loaded with 10 oocytes per lane and is representative of 2 separate experiments conducted in duplicate. The biotinylation assay used material from 3 immunoprecipitations (10 oocytes per IP), and the biotinylated fraction from this pooled material was isolated using strepavidin beads (representative of 2 separate experiments). One-twentieth of the input from the 3 IPs is also shown. Both methods confirm that GPA increases the level of wild-type B3 at the cell surface as previously reported, but only a small amount of S667F-B3 is detected at the cell surface; this does not appear to increase upon coexpression with GPA under the conditions used. (E) Effects of coexpression of 0.75 ng normal B3 with 0.75 ng S667F-B3 on chloride influx. Results are expressed as percentages of the chloride influx obtained with normal B3 and are shown as means plus or minus SEM. The predicted amount of activity is also shown, which represents the expected contribution of 50% B3 and 50% S667F-B3 chloride influx assuming that each population is independent. This result shows that coexpression of wild-type B3 with S667F-B3 rescues the chloride influx of S667F-B3 beyond the predicted level (representative of 4 independent experiments).