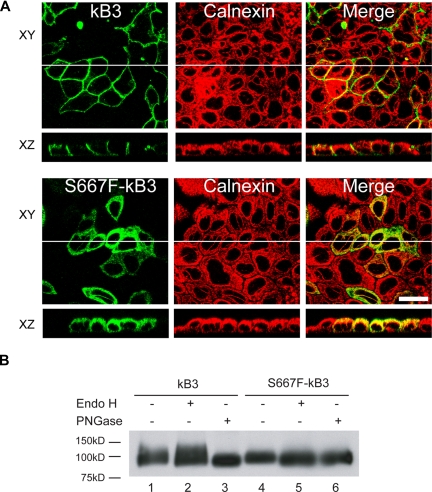
Figure 6.
Polarized expression of S667F-kB3 in MDCK1 cells and endoglycosidases treatment. (A) MDCK1 cells stably expressing kB3 or S667F-kB3 that were allowed to polarize for 3 days; their protein expression was induced with sodium butyrate and fixed as described in “Methods.” The cells were then double-labeled with anti–band 3 mouse monoclonal BRIC170 and a rabbit antibody to calnexin, and the bound antibodies were detected with suitable goat anti-mouse or anti-rabbit secondary antibodies and imaged using confocal microscopy. The top panels (XY) show a view parallel to the epithelium, with the BRIC170 image, calnexin, and merged images. The images below (XZ) show a perpendicular view of BRIC170, calnexin, and the merged image of the same epithelium, as represented by the white line in the XY image. kB3 did not overlap with calnexin distribution, and is localized to the basolateral membrane. In contrast, the majority of the S667F-kB3 immunoreactive protein overlaps with the localization of calnexin in polarized cells. Scale bar equals 30 μM. (B) Western blot of kB3 or S667F-kB3 proteins immunoprecipitated with rbB3Ct from one confluent 10-cm2 plate of cells induced with sodium butyrate and treated with either nothing, Endo H (removes high mannose glycosylation), and PNGase (removes complex glycosylation). The immunoprecipitated proteins were eluted and detected by Western blotting using anti–band 3 BRIC170. Normal kB3 protein is complex glycosylated, as evidenced by a diffuse band present in lane 1, which is insensitive to Endo H treatment (lane 2) but runs as a lower-molecular-weight after treatment with PNGase (lane 3). In contrast, S667F-kB3 does not have a diffuse band (lane 4) and is sensitive to Endo H (compare lane 4 with 5), suggestive of high mannose glycosylation only, and consistent with this protein being retained in the ER.