Abstract
A fully automated procedure, involving computer-controlled stimulus presentation and computer-recorded response measurement, was used for the first time to study imitation in non-human animals. After preliminary training to peck and step on a manipulandum, budgerigars were given a discrimination task in which they were rewarded with food for pecking during observation of pecking and for stepping during observation of stepping (Compatible group), or for pecking while observing stepping and for stepping while observing pecking (Incompatible group). The Incompatible group, which had to counter-imitate for food reward, showed weaker discrimination performance than the Compatible group. This suggests that, like humans, budgerigars are subject to ‘automatic imitation’; they cannot inhibit online the tendency to imitate pecking and/or stepping, even when imitation of these behaviours interferes with the performance of an ongoing task. The difference between the two groups persisted over 10 test sessions, but the Incompatible group eventually acquired the discrimination, making more counter-imitative than imitative responses in the final sessions. These results are consistent with the associative sequence learning model, which suggests that, across species, the development of imitation and the mirror system depends on sensorimotor experience and phylogenetically ancient mechanisms of associative learning.
Keywords: associative sequence learning, automatic imitation, budgerigars, discrimination learning, stimulus–response compatibility, mirror system
1. Introduction
Humans are known to be subject to ‘automatic imitation’; the sight of another person's action tends to elicit the same action from the observer, even when this imitative tendency interferes with efficient performance of an ongoing task (Stürmer et al. 2000; Brass et al. 2001; Kilner et al. 2003). For example, if a person is instructed to open his/her hand as fast as possible whenever he/she sees a hand movement, responses are slower when the stimulus hand closes than when it opens (Heyes et al. 2005). At the neurological level, automatic imitation is thought to be mediated by the ‘mirror system’, areas of the premotor and parietal cortices that have been shown using functional imaging and transcranial magnetic stimulation to be active during passive observation of actions and during execution of the same actions without visual feedback (e.g. Buccino et al. 2001; Gangitano et al. 2004). The occurrence of automatic imitation in everyday life is thought to promote affiliation and cooperation among social partners (Chartrand & Bargh 1999; van Baaren et al. 2004).
The associative sequence learning (ASL) model suggests that automatic imitation and the mirror system develop through sensorimotor learning (Heyes 2001, 2005; see also Keysers & Perrett 2004). The suggestion is that observation of a given action—for example, hand opening—acquires the potential to elicit activation of the same action in the observer through experience in which observation and execution of that action have been correlated and coincident. This kind of experience leads, through associative learning, to the formation of bidirectional excitatory links between sensory and motor representations of the action. For movements of the human hand, this kind of experience—in which action execution is predictive of, and temporally contiguous with, observation of the same action—can be obtained by watching one's own movements.
A broad range of vertebrate and invertebrate species are capable of associative learning (Pearce 2008), and many non-human animals are likely to receive, through self-observation or by other means, correlated experience of observing and executing at least some of their behaviours. Therefore, if the ASL model is correct, one would expect to find automatic imitation in non-human species.
Recent work on the ‘pecking–stepping imitation effect’ suggests, but does not show conclusively, that birds are subject to automatic imitation. When one group of birds has observed a conspecific pecking an object, and a second group has observed a conspecific stepping on the object, the members of both groups typically direct both pecks and steps to the object. However, the proportion of pecking to stepping responses is biased towards pecking in the birds that observed pecking, and towards stepping in the birds that observed stepping (e.g. budgerigars: Dawson & Foss 1965, Richards et al. submitted; pigeons Columba livia: Zentall et al. 1996, Nyuyen et al. 2005, Saggerson et al. 2005, McGregor et al. 2006; quail Coturnix japonica: Akins & Zentall 1996, Dorrance & Zentall 2001).
In all of these studies reporting a pecking–stepping imitation effect, the tested birds are likely to have had experience of feeding in groups. In birds, social foraging provides correlated experience of observing and executing pecking behaviour. Therefore, it is plausible that, as the ASL model suggests, the birds' imitative behaviour in these experiments was due to prior associative learning. However, in all of these studies, the observer birds were rewarded with food for both pecking and stepping, or for neither response, and under these conditions the occurrence of imitation had no impact on reinforcement rate; on the efficiency with which the bird discharged its task of obtaining food. Therefore, previous studies leave open the question of whether, like automatic imitation in humans, the tendency of birds to imitate pecking is so strong that it will interfere with efficient performance of an ongoing task.
In the present study, we tested for automatic imitation in birds using an analogue of the stimulus–response compatibility (SRC) paradigms that are currently used to investigate automatic imitation in humans (e.g. Brass et al. 2001). We compared the behaviour of two groups of observer budgerigars in a pecking–stepping discrimination task. All of the birds observed, on video, a conspecific demonstrator pecking at and stepping on an object. The pecking and stepping stimuli were each presented briefly and in an unpredictable sequence. To obtain food reward, the birds in the Compatible group were required to peck during the pecking stimulus and to step during the stepping stimulus, whereas the birds in the Incompatible group were required to step during the pecking stimulus, and to peck during the stepping stimulus. Under these conditions, imitation would be consistent with correct (rewarded) responding in the Compatible group, and inconsistent with correct responding in the Incompatible group. Therefore, if imitation in birds can interfere with efficient performance of an ongoing task, then the Incompatible group should make fewer rewarded responses than the Compatible group.
2. Material and methods
(a) Subjects
The subjects were 22 adult budgerigars. Their weights ranged from 33 to 60 g. They were housed together in a cage (88 h×40 w×30 d cm) in a holding room with a 12 L : 12 D cycle and a temperature of 19–20°C. The birds had free access to water, cuttlebone, grit and water baths. During the experiment they were weighed daily and maintained at 85 per cent of their free-feeding weights by being fed a restricted amount of food after each experimental session. The food was ‘Budgie Mix’ (H. G. Gladwell & Sons, Ltd, Ipswich, UK), which is a mixture of canary seed and white and red millet. This research adhered to the Association for the Study of Animal Behaviour/Animal Behaviour Society Guidelines for the Use of Animals in Research (published on the Animal Behaviour website), the legal requirements of the country in which the work was carried out and all institutional guidelines.
(b) Apparatus
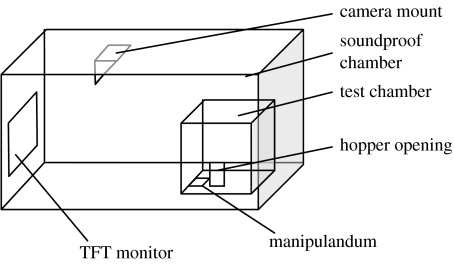
Four conditioning chambers (25×25×25 cm) were housed in separate light- and sound-attenuating chests (78×46×44 cm; figure 1). A colour TFT monitor (ViewSonic, VS10057, 7024×768) with a screen that was 27 cm high and 34 cm wide was attached to the left-hand side wall of each chest. The midpoint of the screen was 20 cm above the floor, 20 cm from the rear wall and 8.5 cm from the left-hand side wall of the chest. The set of four monitors was connected to a Pentium PC through a quad splitter (AVC 704R, Colour Quad Processor). The walls of the conditioning chambers were made from clear Perspex. The wall of each chamber that was nearest to the TFT screen was hinged at the bottom to serve as a door. This door was parallel to the TFT screen and was 40 cm from it. The floors of each chamber were 6 cm above the floor of the chest. During the experiment, Budgie Mix was made available by a grain dispenser (Colbourn Instruments, Lehigh Valley, PA) that was attached to the wall to the left of the door of the conditioning chamber. The grain feeder had an opening that was 6×7 cm. The midpoint of the opening was 3.5 cm above the floor of the chamber, and 7 cm from the door.
Figure 1.
A diagram of the apparatus.
The response key consisted of a round Perspex box with a diameter of 3 cm, which was located on the floor of the conditioning chamber. The top of the lid was 0.5 cm above the floor of the chamber and its midpoint was 3 cm from the door, and 12 cm from the wall containing the grain feeder. The round lid was surrounded by a metal rim with a diameter of 3.2 cm. The lid was semi-transparent. A microswitch operated whenever a force of greater than 6 g was applied to the lid.
Illumination in the conditioning chambers was provided by the events on the TFT screen, and by a bulb in the grain feeder that was turned on whenever grain was made available. The presentation of stimuli on the TFT screens, the recording of responses and the operation of the grain feeder were controlled by a PC (Research Machines, Abingdon, UK) running Windows XP. The computer was programmed in VisualBasic and the interface with the experimental apparatus was controlled by Whisker software (Campden Instruments Ltd, Loughborough, UK).
During the experiment, the doors of the light- and sound-attenuating chests and the conditioning chambers were closed. The subjects were viewed by a camera mounted on the ceiling. From the camera perspective, the hopper was on the right-hand side wall of the test chamber.
(c) Video stimuli
Two video clips were used—one showing a budgerigar pecking the manipulandum and another showing the same bird stepping on the manipulandum (figure 2). Both clips were recorded using a digital video camera (Sony Handycam DCR-HC30E) and edited using Adobe Premiere Pro v. 1.5. The footage was filmed so that the images played on the TFT screen were life size. The camera was placed outside the testing chamber, between the monitor screen and the front wall of the testing chamber. The camera was fitted on a tripod, facing towards the chamber, where from the camera perspective, the hopper was on the right. For the purposes of creating the video clip of stepping, one instance in which the foot was lifted from the floor of the chamber and placed on the manipulandum was selected from a 30 min recording made in the single recording session. During video editing, the original instance of the selected response was played forward and then in reverse to create a smooth single step on the manipulandum. The speed of the clip, which consisted of the foregoing sequence repeated twice, was adjusted to make it last for a total of 2.14 s. The clip was then looped 56 times to make 112 steps in the 2 min video (step video). Thus, the step video showed a bird standing upright, which then placed its right foot on the manipulandum with its body shifted a little, and then drew its foot back to the original position with the body also shifted back. A method similar to that just described was adopted in order to create a 2 min (112 pecks) video of the demonstrator pecking the manipulandum (peck video).
Figure 2.
Stills from the stimulus videos of (a) pecking and (b) stepping.
In each session, the peck and step videos were each presented seven times, 2 min at a time, in a random sequence with the constraint that the same clip was not shown more than twice in succession. There was an interval of 20 s before the first video clip was shown and there was an interval of 10 s between successive clips. The TFT screens were entirely white during these intervals. Food was made available for pecking or stepping on the response key according to a variable interval (VI) 15 s schedule during the clip of the demonstrators either pecking or stepping.
(d) Procedure
(i) Preliminary training
Throughout the experiment, every time that the microswitch on the response key was operated, a record was taken of the duration for which it was closed. A peck was deemed to have been responsible for this closure if the duration of closure was less than 0.100 s. If this duration was greater than 0.100 s then the response was classified as a step. These values were selected on the basis of pilot work with three budgerigars that were hand shaped to both peck and step on the response key to gain food. For two birds, pecks were rewarded when the entire television screen was red, and steps were rewarded when the entire television screen was green. For the remaining bird, pecks were rewarded during the green light, and steps during the red light. This training continued until more than 90 per cent of the responses made during each stimulus were correct. We then recorded during a 1 min presentation of each stimulus, the duration of each response. During the stimulus that signalled the availability of food for pecking, the three birds made a total of 67 responses. If one outlier response was removed (0.31 s), the mean duration of the remaining responses was 0.038 s (range: 0.008–0.102) and the standard deviation was 0.020. During the stimulus that signalled the availability of food for stepping, 89 responses were recorded. If one outlier response was removed (2.03 s), the mean duration of the remaining responses was 0.259 s (range: 0.090–0.879 s) and the standard deviation was 0.144. There were two responses during the stimulus in which pecking was rewarded which were longer than the 0.10 s criterion, and three responses during the stimulus in which steps were rewarded which were shorter than 0.10 s.
After being trained to feed from the food hopper (21–35 days), the birds were trained to peck and step on the manipulandum to operate the hopper. Both responses were then reinforced on a continuous schedule for 2 days, and then on a gradually increasing VI schedule until, after 20 days, the birds were performing reliably on a VI 15 s schedule. On a VI 15 s schedule the subject is rewarded with food for a correct response with an average interval of 15 s.
(ii) Red–green discrimination task
To familiarize the birds with the procedure to be used in the peck–step discrimination task, and to make sure that the birds assigned to the Compatible and Incompatible groups did not differ in their discrimination learning ability, all the birds were given red–green discrimination training. In each session there were nine trials in which the screen was illuminated green for 1 min, and nine trials where it was illuminated with red. The sequence of the illumination was randomized. The screen was dark for an interval of 10 s between each trial. Eleven subjects (six in the Compatible group and five in the Incompatible group) were trained to peck in order to gain food delivered according to a VI 15 s schedule when the TFT screen was green and to step for food when it was red. Pecks during red and steps during green were without programmed consequences. The remaining 11 subjects were trained in a manner opposite to that just described.
(iii) Peck–step discrimination task
Two days after the completion of red–green discrimination training, the birds received 13 sessions of peck–step discrimination training. The observers were divided equally into two groups (Compatible group and Incompatible group). Subjects in the Compatible group were rewarded with food on a VI 15 s schedule when they performed the same response as the demonstrator (peck when the video of pecking was presented; step when the video of stepping was presented). For subjects in the Incompatible group, performing the response opposite to that being demonstrated was reinforced, also according to VI 15 s schedule. That is, food was presented when subjects pecked at the response key during the stepping video, and stepped on the key during the pecking video. In each trial, we recorded the total number of correct and of incorrect responses that were made. Correct responses were those that could potentially result in the delivery of food, whereas incorrect responses were those that could not result in the delivery of food.
3. Results
To compare the performance of the Compatible and Incompatible groups on the red–green discrimination, for each subject, the mean number of correct responses that were made during the red and green trials, and the mean number of incorrect responses were calculated for the final two sessions of red–green training. The group means (and standard error scores) were the following: Compatible group, correct—137.56 (27.5); Compatible group, incorrect—96.31 (24.73); Incompatible group, correct—168.41 (27.67); and Incompatible group, incorrect—104.27 (25.24). Two-way analysis of variance (ANOVA) revealed a significant effect of response (correct or incorrect; F1,20=32.55, p=0.001), indicating that the birds acquired the discrimination. However, the effect of group (F1,20=2.06) and the Group×Response interaction (F1,20<1) were not significant, confirming that the Compatible and Incompatible groups did not differ in their red–green discrimination performance.
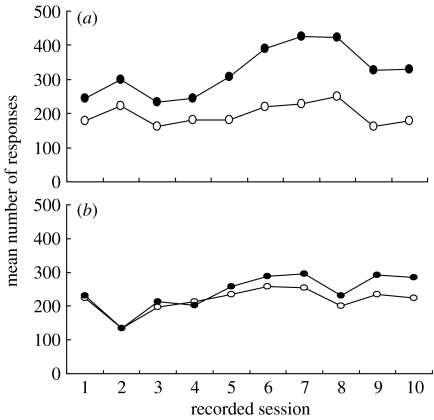
The results from the test phase of the experiment are shown in figure 3. The data from the first three sessions are not included because they were lost due to an error in the computer program that recorded responses. Figure 3a shows the mean rates at which the correct and incorrect responses were performed by the Compatible group. Figure 3b shows the equivalent results for the Incompatible group. From these graphs, it is evident that the Compatible group made more correct responses than incorrect responses throughout the 10-session test period. By contrast, the Incompatible group made the same number of correct and incorrect responses during the first few sessions of training, and only gradually developed some tendency to make more correct responses than incorrect ones. Thus, the discrimination performance of the Incompatible group was substantially weaker than that of the Compatible group.
Figure 3.
Mean number of correct (filled circles) and incorrect (open circles) responses by birds in the (a) Compatible and (b) Incompatible groups over the test sessions.
These impressions were confirmed by ANOVA in which response type (correct or incorrect), stimulus video (peck or step) and session (1–10) were within-subjects factors, and group (Compatible or Incompatible) was the between-subjects factor. This revealed a significant main effect of response type (F1,20=12.26, p=0.002), indicating that there were more correct responses than incorrect responses. It also yielded a significant main effect of session (F9,180=3.53, p=0.004), and a significant response type–session interaction (F9,180=2.93, p=0.018), showing that the number of responses per session increased over sessions, and that this trend was more pronounced for correct responses than for incorrect ones. Of principal interest, the analysis revealed a significant response type by group interaction (F1,20=7.53, p=0.013), confirming that the discrimination performance of the Incompatible group was inferior to that of the Compatible group. No other main effects or interactions (two-, three- or four-way) were significant.
In spite of their weaker performance, the Incompatible group did eventually acquire the peck–step discrimination; across the last three sessions of testing, they made more correct responses than incorrect responses (F1,10=17.36, p=0.002).
4. Discussion
The results of the present study provide the first evidence of automatic imitation in birds. Over 10 recorded sessions of discrimination training, budgerigars that were required not to imitate in order to gain food reward, i.e. to peck while observing stepping and to step while observing pecking (Incompatible group), made fewer correct responses relative to incorrect ones than budgerigars that were required to imitate for food, i.e. to peck while observing pecking and to step while observing stepping (Compatible group).
In the majority of previous studies of imitative pecking and stepping, observer birds saw rewarded pecking or rewarded stepping, and were themselves rewarded with equal frequency for pecking and stepping (e.g. Dawson & Foss 1965; Akins & Zentall 1996; Zentall et al. 1996; Richards et al. submitted). These studies were important in establishing that a pecking–stepping imitation effect can be reliably detected in a variety of bird species, but they did not address the question of whether imitation in birds is goal directed. More specifically, they did not investigate whether imitation in birds depends on action–outcome learning by observation (e.g. learning that pecking is followed by the delivery of food), and whether imitative responses are made in the expectation that they will be rewarded (but see Dorrance & Zentall 2001; Saggerson et al. 2005). One previous study (McGregor et al. 2006) found a pecking–stepping imitation effect in pigeons when neither the observers nor the demonstrators were rewarded for pecking or for stepping. This suggested that imitative behaviour in birds is not goal directed; that they will imitate in the absence of any extrinsic reward for imitation, and when they have not seen the demonstrator's responses being rewarded. However, unlike the present study, McGregor et al. did not show that birds will imitate even when imitation is costly, when it reduces the rate at which they can obtain food. The birds in the Incompatible group were tested under these conditions, and yet, when contrasted with the birds in the Compatible group, they continued to provide evidence of imitation throughout the experiment. This suggests that, at least in the context of the pecking–stepping imitation effect, imitation in birds is automatic or involuntary and that it cannot be inhibited by mechanisms that are sensitive to behavioural outcomes.
The automatic imitation effect found in the present study is analogous to those found in human participants using SRC paradigms (e.g. Stürmer et al. 2000). Using within-subjects designs, the human studies show that responding in movement incompatible trials (e.g. a hand opening response to a hand closing stimulus) is slower than responding in movement compatible trials (e.g. a hand opening response to a hand opening stimulus). Similarly, but using a between-subjects design, the present study shows that birds required to make movement incompatible responses in every trial respond less accurately than birds required to make movement compatible responses. The fact that automatic imitation occurs not only in birds but also in humans suggests that it is mediated by phylogenetically general mechanisms. The ASL model suggests that these are the mechanisms of associative learning, and that they produce automatic imitation by establishing, on the basis of correlated experience of observing and executing the same action, a ‘matching vertical association’—an excitatory link between sensory and motor representations of that action (Heyes 2001, 2005).
The ASL model implies that, when associative learning has established a matching vertical association for a given action, X, then activation of the sensory representation of X by perception of X will necessarily be propagated to the motor representation of X (Heyes & Bird 2007). This propagation cannot be prevented ‘at will’, by online control mechanisms, and in this sense imitation is automatic. However, the model does not suggest that, once established, matching vertical associations are immutable. On the contrary, it assumes that experience in which observation of one action, X, is reliably correlated with execution of a different action, Y, will lead to the formation of a new, non-matching vertical association between the sensory representation of X and the motor representation of Y, and to inhibitory links between the sensory and motor representations of X. As a consequence of this learning, automatic imitation of X should decline and ultimately be replaced by automatic counter-imitation—an involuntary tendency for observation of X to elicit execution of Y. This prediction has been tested and confirmed in studies showing that, in humans, automatic imitation effects can be enhanced by compatible sensorimotor training (Press et al. 2007), and abolished (Heyes et al. 2005) or even reversed (Catmur et al. 2007) by incompatible sensorimotor training. The present study was not designed to test this prediction, but the results provide some evidence that, by the end of peck–step discrimination training, the birds in the Incompatible group, which had been compelled by the experimental contingencies to peck when they saw stepping and vice versa, were beginning to make more correct, counter-imitative responses than incorrect, imitative responses. To find out whether incompatible training can result in a full reversal of the pecking–stepping imitation effect, it would be necessary not only to continue this training regime for many more sessions but also to prevent the birds from feeding in groups, and thereby receiving further compatible training, in their home cages between experimental sessions.
Like the ASL model, the ‘response facilitation’ hypothesis (e.g. Byrne 1994) assumes that, for example, the sight of pecking involuntarily activates or ‘primes’ a motor representation or ‘brain record’ of pecking. However, whereas the ASL model suggests that the potential for priming depends on learning, and specifically on temporal contiguity and contingency, the response facilitation hypothesis suggests that it depends on similarity. According to this view, the sight of another bird engaging in a behaviour, B, will activate a motor representation of B in the observer to the extent that B looks the same when observed and executed. The results of the present study do not distinguish between the ASL and response facilitation accounts of automatic imitation, but those of a parallel study (Richards et al. submitted) favour the associative account. They show that the pecking–stepping imitation effect in budgerigars persists even when there is a 24 hour delay between demonstrator observation and behavioural testing. This is inconsistent with the response facilitation hypothesis because it emphasizes the transitory nature of similarity-based priming, and distinguishes it firmly from ‘imitation’ or response learning by observation (Heyes 1994). By contrast, the ASL model suggests not only that automatic imitation is a product of learning, but also that one of its primary functions is to provide the basis for further imitative learning (Heyes & Ray 2000). According to this model, a necessary condition for acquiring new responses by observation (imitation learning) is the establishment of a repertoire of matching vertical associations, each capable of priming of a response that is already in the animal's repertoire (automatic imitation).
The analysis of the data from the current experiment did not reveal an asymmetry between automatic imitation of pecking and stepping; the ANOVA did not yield a significant main effect of stimulus (pecking versus stepping), or any significant interactions involving this variable. However, visual inspection of the data suggested that the tendency to imitate pecking was stronger than the tendency to imitate stepping, and this pattern is consistent with the results of a recent study that has found reliable imitation of pecking, but not of stepping, in budgerigars (Richards et al. submitted). It has been known for a long time that, in birds, observation of pecking increases the probability of pecking behaviour (e.g. Turner 1964; Tolman & Wilson 1965), and it has often been assumed that this tendency, described as ‘contagion’ or ‘social facilitation’, is innate. The present study does not resolve the question of whether imitative pecking is innate or, as the ASL hypothesis suggests, a result of learning. However, two related points are worth noting. First, the ASL model predicts that birds will have a stronger tendency to imitate pecking than to imitate stepping (Richards et al. submitted), whereas the alternative contagion account merely offers a post hoc explanation for this asymmetry. Second, although it has been widely assumed that imitation of pecking is innate, there is not, as far as we are aware, any compelling evidence to support this view.
In humans, automatic imitation is thought to be mediated at the neurological level by the mirror system, areas of premotor and parietal cortices that are active during passive observation of actions and during execution of the same actions without visual feedback (Brass & Heyes 2005). Therefore, although Tchernichovski & Wallman (2008) examined auditory–motor, rather than visual–motor, matching, the results of the present study accord well with their recent discovery of ‘mirror neurons’ in birds (Tchernichovski & Wallman 2008).
As far as we are aware, the present study is the first to use a fully automated procedure to investigate imitation in non-human animals. Some previous studies of imitation in birds have used computer-controlled video stimuli (Richards et al. submitted), or computer-recorded measures of response type (Saggerson et al. 2005), but they have not combined these techniques. A fully automated procedure allows both precise stimulus control and reliable, impartial response measurement. It is, perhaps, appropriate that the first study combining these strengths should find evidence of ‘automatic’ imitation in birds.
Acknowledgments
This research adhered to the Association for the Study of Animal Behaviour/Animal Behaviour Society Guidelines for the Use of Animals in Research (published on the Animal Behaviour website), the legal requirements of the country in which the work was carried out and all institutional guidelines.
This work was supported by the Biotechnology and Biological Sciences Research Council.
References
- Akins C.K, Zentall T.R. Imitative learning in male Japanese quail (Coturnix japonica) using the two-action method. J. Comp. Psychol. 1996;110:316–320. doi: 10.1037/0735-7036.110.3.316. doi:10.1037/0735-7036.110.3.316 [DOI] [PubMed] [Google Scholar]
- Brass M, Heyes C. Imitation: is cognitive neuroscience solving the correspondence problem? Trends Cogn. Sci. 2005;9:489–495. doi: 10.1016/j.tics.2005.08.007. doi:10.1016/j.tics.2005.08.007 [DOI] [PubMed] [Google Scholar]
- Brass M, Bekkering H, Prinz W. Movement observation affects movement execution in a simple response task. Acta Psychol. 2001;106:3–22. doi: 10.1016/s0001-6918(00)00024-x. doi:10.1016/S0001-6918(00)00024-X [DOI] [PubMed] [Google Scholar]
- Buccino G, et al. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur. J. Neurosci. 2001;13:400–404. doi:10.1046/j.1460-9568.2001.01385.x [PubMed] [Google Scholar]
- Byrne R.W. The evolution of intelligence. In: Slater P.J.B, Halliday T.R, editors. Behaviour and evolution. Cambridge University Press; Cambridge, UK: 1994. pp. 223–265. [Google Scholar]
- Catmur C, Walsh V, Heyes C.M. Sensorimotor learning configures the human mirror system. Curr. Biol. 2007;17:1527–1531. doi: 10.1016/j.cub.2007.08.006. doi:10.1016/j.cub.2007.08.006 [DOI] [PubMed] [Google Scholar]
- Chartrand T.L, Bargh J.A. The chameleon effect: the perception–behaviour link and social interaction. J. Pers. Soc. Psychol. 1999;76:893–910. doi: 10.1037//0022-3514.76.6.893. doi:10.1037/0022-3514.76.6.893 [DOI] [PubMed] [Google Scholar]
- Dawson B.V, Foss B.M. Observational learning in budgerigars. Anim. Behav. 1965;13:470–474. doi: 10.1016/0003-3472(65)90108-9. doi:10.1016/0003-3472(65)90108-9 [DOI] [PubMed] [Google Scholar]
- Dorrance B.R, Zentall T.R. Imitative learning in Japanese quail depends on the motivational state of the observer quail at the time of observation. J. Comp. Psychol. 2001;115:62–67. doi: 10.1037/0735-7036.115.1.62. doi:10.1037/0735-7036.115.1.62 [DOI] [PubMed] [Google Scholar]
- Gangitano M, Mattaghy F.M, Pascual-Leone A. Modulation of premotor mirror neuron activity during observation of unpredictable grasping movements. Eur. J. Neurosci. 2004;20:2193–2202. doi: 10.1111/j.1460-9568.2004.03655.x. doi:10.1111/j.1460-9568.2004.03655.x [DOI] [PubMed] [Google Scholar]
- Heyes C.M. Social learning in animals: categories and mechanisms. Biol. Rev. 1994;69:207–231. doi: 10.1111/j.1469-185x.1994.tb01506.x. doi:10.1111/j.1469-185X.1994.tb01506.x [DOI] [PubMed] [Google Scholar]
- Heyes C.M. Causes and consequences of imitation. Trends Cogn. Sci. 2001;5:253–261. doi: 10.1016/s1364-6613(00)01661-2. doi:10.1016/S1364-6613(00)01661-2 [DOI] [PubMed] [Google Scholar]
- Heyes C.M. Imitation by association. In: Hurley S, Chater N, editors. Perspectives on imitation: from cognitive neuroscience to social science. MIT Press; Cambridge, MA: 2005. pp. 157–176. [Google Scholar]
- Heyes C.M, Bird G. Mirroring, association and the correspondence problem. In: Haggard P, Rossetti Y, Kawato M, editors. Sensorimotor foundations of higher cognition, attention and performance XX. Oxford University Press; Oxford, UK: 2007. pp. 461–479. [Google Scholar]
- Heyes C.M, Ray E.D. What is the significance of imitation in animals? Adv. Study Behav. 2000;29:215–245. doi:10.1016/S0065-3454(08)60106-0 [Google Scholar]
- Heyes C.M, Bird G, Johnson H, Haggard P. Experience modulates automatic imitation. Cogn. Brain Res. 2005;22:233–240. doi: 10.1016/j.cogbrainres.2004.09.009. doi:10.1016/j.cogbrainres.2004.09.009 [DOI] [PubMed] [Google Scholar]
- Keysers C, Perrett D.I. Demystifying social cognition: a Hebbian perspective. Trends Cogn. Sci. 2004;8:501–507. doi: 10.1016/j.tics.2004.09.005. doi:10.1016/j.tics.2004.09.005 [DOI] [PubMed] [Google Scholar]
- Kilner J.M, Paulignan Y, Blakemore S.J. An interference effect of observed biological movement on action. Curr. Biol. 2003;13:522–525. doi: 10.1016/s0960-9822(03)00165-9. doi:10.1016/S0960-9822(03)00165-9 [DOI] [PubMed] [Google Scholar]
- McGregor A, Saggerson A, Pearce J.M, Heyes C.M. Blind imitation in pigeons, Columba livia. Anim. Behav. 2006;72:287–296. doi:10.1016/j.anbehav.2005.10.026 [Google Scholar]
- Nyuyen N.H, Klein E.D, Zentall T.R. Imitation of a two-action sequence in pigeons. Psychon. Bull. Rev. 2005;12:514–518. doi: 10.3758/bf03193797. [DOI] [PubMed] [Google Scholar]
- Pearce J.M. Psychology Press; Hove, UK: 2008. Animal learning and cognition: an introduction. [Google Scholar]
- Press C, Gillmeister H, Heyes C.M. Sensorimotor experience enhances automatic imitation of robotic action. Proc. R. Soc. B. 2007;274:2509–2514. doi: 10.1098/rspb.2007.0774. doi:10.1098/rspb.2007.0774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards, C., Mottley, K., Pearce, J. M. & Heyes, C. M. Submitted. Imitative pecking by budgerigars over a 24-hour delay.
- Saggerson A.L, George D.N, Honey R.C. Imitative learning of stimulus–response and response–outcome associations in pigeons. J. Exp. Psychol. Anim. Behav. Process. 2005;31:289–300. doi: 10.1037/0097-7403.31.3.289. doi:10.1037/0097-7403.31.3.289 [DOI] [PubMed] [Google Scholar]
- Stürmer B, Aschersleben G, Prinz W. Effects of correspondence between complex stimulus and response patterns. J. Exp. Psychol. Hum. Percept. Perform. 2000;26:1746–1759. doi: 10.1037//0096-1523.26.6.1746. doi:10.1037/0096-1523.26.6.1746 [DOI] [PubMed] [Google Scholar]
- Tchernichovski O, Wallman J. Neurons of imitation. Nature. 2008;451:249–250. doi: 10.1038/451249a. doi:10.1038/451249a [DOI] [PubMed] [Google Scholar]
- Tolman C.W, Wilson G.F. Social feeding in domestic chicks. Anim. Behav. 1965;13:134–142. doi: 10.1016/0003-3472(65)90111-9. doi:10.1016/0003-3472(65)90083-7 [DOI] [PubMed] [Google Scholar]
- Turner E.R.A. Social feeding in birds. Behaviour. 1964;25:1–43. doi:10.1163/156853964X00201 [Google Scholar]
- van Baaren R.B, Holland R.W, Kawakami K, van Knippenberg A. Mimicry and prosocial behavior. Psychol. Sci. 2004;15:71–74. doi: 10.1111/j.0963-7214.2004.01501012.x. doi:10.1111/j.0963-7214.2004.01501012.x [DOI] [PubMed] [Google Scholar]
- Zentall T.R, Sutton J.E, Sherburne L.M. True imitative learning in pigeons. Psychol. Sci. 1996;7:343–346. doi:10.1111/j.1467-9280.1996.tb00386.x [Google Scholar]