Abstract
Theoreticians have long suggested that the amount of intergroup conflict in which a group is involved could influence the level of cooperation or affiliation displayed by its members. Despite the prevalence of intergroup conflicts in many social animal species, however, few empirical studies have investigated this potential link. Here, I show that intragroup allopreening rates are highest in green woodhoopoe (Phoeniculus purpureus) groups that have the greatest involvement in intergroup conflict. One reason for this relationship is a post-conflict increase in allopreening, and I demonstrate for the first time that both conflict duration and outcome influence subsequent allopreening rates: group members allopreened more following long conflicts and those they lost compared with short conflicts and those they won, perhaps because the former are more stressful. The increase in affiliative behaviour was the result of more allopreening of subordinate helpers by the dominant breeding pair, which may be because the breeders are trying to encourage helpers to participate in future conflicts; relative group size influences conflict outcome and helpers contribute more to conflicts than do the breeding pair. These results emphasize that our understanding of cooperation and group dynamics can be enhanced by investigations of how intergroup interactions affect intragroup processes.
Keywords: allopreening, cooperation, intergroup conflict, reconciliation, social behaviour, stress
1. Introduction
In many social species, including humans, conflict between groups (intergroup conflict) is commonplace (Radford 2003; Choi & Bowles 2007; Kitchen & Beehner 2007). For example, group members often produce a combined display (McComb et al. 1994; Radford 2003) or fight alongside one another (Watts & Mitani 2001; Wilson et al. 2001) when defending their territory against rival groups. Theoreticians have long suggested that the amount of intergroup conflict in which a group is involved could influence the amount of cooperation or affiliation displayed by its members (Hamilton 1975; Alexander & Borgia 1978). Selection for cooperation should be reduced when intergroup conflict occurs at a low rate relative to conflict between group members (intragroup conflict), and this is true whether groups are composed of relatives (West et al. 2002) or non-relatives (West et al. 2006). Increased intergroup conflict should favour higher levels of cooperation, especially if cohesion between group members is important for success (Reeve & Hölldobler 2007). Despite these clear predictions, empirical investigations of the relationship between intergroup conflict and intragroup affiliative behaviour are rare in non-human animals (for exceptions, see Cheney 1992; Radford 2008).
Within a particular species, intergroup conflicts can vary greatly in their duration, intensity and outcome (Radford & du Plessis 2004a; Wich & Sterck 2007). Moreover, individual group members often differ in their contributions to these conflicts depending on, for example, their age, sex and dominance status (Heinsohn & Packer 1995; Cant et al. 2002; Kitchen & Beehner 2007). Several studies of intragroup conflict have shown that the characteristics of both the conflict and those involved can affect the level of affiliative behaviour subsequently displayed (Schino et al. 1998; Koski et al. 2007). For example, allogrooming, which can strengthen bonds between individuals and reduce stress (Dunbar 1991; Aureli et al. 1999), occurs at a higher rate following intragroup conflicts of greater intensity and those involving same-sex individuals (Schino et al. 1998). To date, no studies have investigated whether the characteristics of an intergroup conflict and its participants similarly influence post-conflict affiliative behaviour.
The cooperatively breeding green woodhoopoe (Phoeniculus purpureus) provides an ideal opportunity to investigate the influence of intergroup conflict on intragroup affiliative behaviour. Woodhoopoes live in groups of 2–8 individuals that defend a territory throughout the year (Radford & du Plessis 2004b). Intragroup conflict is rare; there is a strict queuing system for breeding positions (Ligon & Ligon 1990) and vocalizations are used to maintain spacing between potential foraging competitors (Radford 2004a). However, neighbouring groups come into conflict several times a day, when they produce raucous vocal displays in which all adult group members participate (Radford 2003). The duration of these vocal conflicts is highly variable, lasting between 1 and 45 min, and the winners and losers can be readily assigned (Radford & du Plessis 2004a). Allopreening, the avian equivalent of allogrooming, is a common and easily scored affiliative behaviour and is known to play an important social role in green woodhoopoes (Radford & du Plessis 2006).
Here, I consider four main questions. First, is intragroup affiliative behaviour (specifically allopreening) more common in groups that spend more time in conflict with their neighbours? Second, is there an increase in intragroup affiliative behaviour following intergroup conflict, as is often the case following intragroup conflict (Aureli et al. 2002)? Third, do the duration and the outcome of intergroup conflicts influence subsequent intragroup allopreening rates? Allopreening is predicted to increase more following those conflicts that are likely to have been more stressful (i.e. those that are longer in duration and that are lost). Fourth, how do group members of different sex and dominance status adjust their allopreening following intergroup conflict? Males and females are predicted to show similar changes in allopreening because they contribute equally to intergroup conflicts (Radford 2003); the dominant breeding pair and subordinate non-breeding helpers may increase their allopreening to different extents because the latter expend more effort in intergroup conflicts (Radford 2003).
2. Material and methods
(a) Study species
Fieldwork on a colour-ringed population of green woodhoopoes was carried out near Morgan's Bay (32°43′ S, 28°19′ E), Eastern Cape Province, South Africa. In this population, 57 per cent of groups have at least one non-breeding helper in addition to the breeding pair (Radford & du Plessis 2004b). The helpers are related to one or both of the breeders in approximately 90 per cent of cases; helping behaviour is unrelated to natal philopatry, kinship or prior association with breeders (du Plessis 1993). Adults can be sexed on the basis of sexual dimorphism in bill length (Radford & du Plessis 2003) and vocalizations (Radford 2004b). Dominance status can be established during foraging, when the members of the putative breeding pair displace same-sex helpers (Radford & du Plessis 2003). Extra-pair paternity in the study population is likely to be very low, as no extra-pair young were identified in the breeding attempts of 16 groups (M. A. du Plessis 1999, unpublished data).
All group members participate in allopreening, which involves one woodhoopoe bringing its bill into firm contact with the feathers of another individual in a preening motion. Allopreening bouts focus on either body parts inaccessible to the recipient itself (i.e. the head and neck; hereafter, the head) or those accessible to the recipient (i.e. lower than the neck; hereafter, the body). Head allopreening serves a primarily hygienic function: it occurs at a constant rate throughout the year; it is highly reciprocated and all group members donate and receive similar amounts (Radford & du Plessis 2006). Body allopreening serves a primarily social function: its rate varies seasonally; it occurs more often in larger groups and the frequency with which bouts are received, donated and reciprocated depends on the dominance status of the participants (Radford & du Plessis 2006).
Groups defend year-round territories in thickly forested riverine valleys (Radford & du Plessis 2004b). When one group (the intruder) trespasses into the territory of another (the resident), and the two groups meet, a conflict arises in which the rivals give alternating raucous vocal displays (rallies); only very rarely do these escalate to physical fighting (Radford & du Plessis 2004a). All adult group members participate in group rallies, but contributions differ depending on the individual's sex and dominance status: males and females contribute equally overall, but each sex expends more effort responding to intruders of its own sex; helpers contribute more than the breeding pair (Radford 2003). Although the territory holders may be usurped by groups from further afield (Ligon & Ligon 1990), conflicts between neighbouring groups do not tend to result in permanent changes in territory size (Radford & du Plessis 2004b). However, intruding neighbours that win a conflict do remain on the resident's territory for up to an hour to forage and examine roost/nest holes, before returning to their own territory (Radford & du Plessis 2004a).
(b) Data collection
Between November 2000 and May 2001, I collected data on allopreening and intergroup conflicts from 12 focal groups (mean±s.e.m. adult group size=3.3±0.3, range 2–5; mean±s.e.m. observation time per group=32.3±3.3 hours, range 18.5–49.2 hours). These groups were chosen because they each bordered two others in the study population. The composition of each focal group, as well as each group member's dominance status, remained constant throughout the data collection period.
I recorded the occurrence of each allopreening bout and the individuals involved. Donors were birds preening others, while recipients were those preened. Bouts were considered finished when one individual moved away from the other or when no allopreening had occurred for 30 s; subsequent bouts were treated as separate events. Because juvenile woodhoopoes (identifiable from their black bills) rarely allopreen (Radford & du Plessis 2006), I consider only interactions between adults (individuals above 12 months old). Because head allopreening serves a primarily hygienic function (see above) and its rate did not increase following experimentally simulated intergroup conflict (Radford 2008), I focus on body allopreening in this paper (mean±s.e.m. bouts per group=49.9±4.4, range 30–85).
I recorded the duration and the outcome of all intergroup conflicts between the 12 focal groups and both their neighbours (mean±s.e.m. conflicts per group=11.5±0.9, range 8–18). An intergroup conflict was deemed to have occurred if the resident responded within 5 min to a rally from the intruder, and was considered finished once no rally had been given for 5 min. If the intruder remained on the resident's territory for at least 10 min after the final rally, and the resident moved deeper into its own territory, the contest was lost by the resident; if the intruder retreated back to its own territory within 1 min of the final rally, the contest was won by the resident (see Radford & du Plessis 2004a). All focal groups won at least some conflicts with each of their two neighbours (mean±s.e.m. conflicts won=54.1±5.3%, range 36.4–73.7%, n=24 focal group/neighbour pairs).
(c) Statistical analysis
Statistical analyses were two tailed and conducted using Genstat (10th edition, Lawes Agricultural Trust, Rothampstead, UK). General linear models (GLMs) were used to assess the body allopreening rates of different groups in relation to their overall involvement in intergroup conflict. However, subsequent analyses included repeated measures of the same group and individual. So, linear mixed models (LMMs), with a normal error structure and an identity link function, and generalized linear mixed models (GLMMs), with a Poisson error structure and a log link function, were used to allow the inclusion of both random and fixed terms (see electronic supplementary material for further details).
To assess whether intragroup body allopreening increased following intergroup conflict, I used a LMM based on 201 rates from 12 groups. I compared allopreening in the hour following intergroup conflict (post-conflict hours) with allopreening in the hour following at least one hour without intergroup conflict (non-conflict hours). To assess whether the duration and the outcome (won and lost) of the intergroup conflicts influenced subsequent intragroup allopreening rates, I used a LMM based on 125 post-conflict body allopreening rates from 12 groups. To assess whether group members of different sex and dominance status (the breeding pair and helpers) altered their post-conflict allopreening to the same extent following conflicts of different outcome, I used two GLMMs (one for allopreening donation and one for receipt) based on 168 changes in hourly body allopreening rate (post-conflict hour rate minus pre-conflict hour rate) from 36 individuals in 10 groups (two groups contained no helpers). In all models, I included group size and month as fixed terms because they are known to influence the rate of body allopreening (Radford & du Plessis 2006).
3. Results
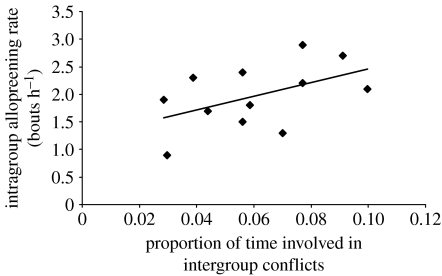
Groups that were involved in intergroup conflict a greater proportion of the time had significantly higher rates of intragroup body allopreening (GLM: F1,9=9.24, p=0.014; figure 1), after controlling for a significant influence of group size (F1,9=9.98, p=0.012). The relationship with intragroup body allopreening rate remained qualitatively the same if the rate at which the intergroup conflicts occurred, rather than the proportion of time involved, was included as a predictor variable (intergroup conflict rate: F1,9=12.22, p=0.007; group size: F1,9=5.79, p=0.039). One reason for the significant positive relationship between intergroup conflict involvement and affiliative behaviour is that intragroup body allopreening was significantly higher in post-conflict hours (mean±s.e.m.=2.45±0.16 bouts per hour) compared with non-conflict hours (0.86±0.12 bouts per hour; LMM: Wald statistic=102.90, d.f.=1, p<0.001), after controlling for significant influences of group size and month (see table 1 in the electronic supplementary material).
Figure 1.
Intragroup body allopreening rates of 12 green woodhoopoe groups in relation to the proportion of observation time involved in intergroup conflict. Least-squares regression line is shown.
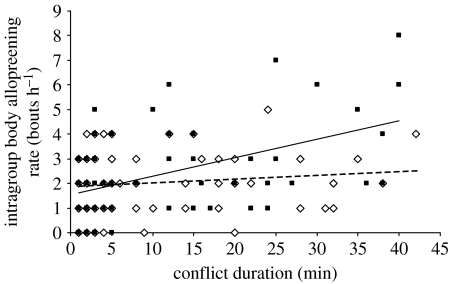
Post-conflict intragroup body allopreening rate was significantly influenced by the interaction between conflict duration and outcome (LMM: Wald statistic=4.90, d.f.=1, p=0.029), after controlling for significant influences of group size and month (see table 2 in the electronic supplementary material). There was no significant difference in body allopreening following winning and losing conflicts of short duration, but there was significantly more body allopreening following long conflicts that were lost compared with those that were won (figure 2).
Figure 2.
Intragroup body allopreening rates of 12 green woodhoopoe groups following different intergroup conflicts. Least-squares regression lines are shown: won conflicts (dashed line, diamonds) and lost conflicts (solid line, squares).
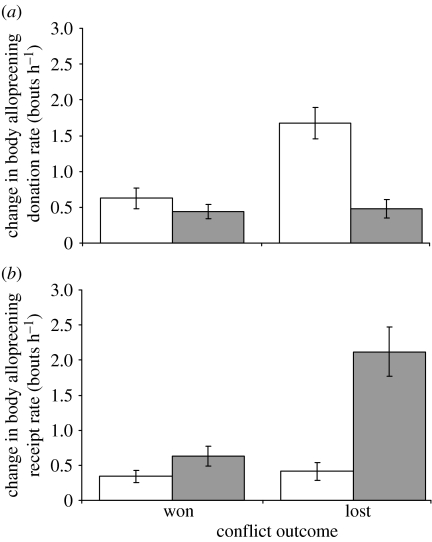
Post-conflict increases in individual body allopreening donation (GLMM: Wald statistic=4.36, d.f.=1, p=0.040) and receipt (Wald statistic=4.41, d.f.=1, p=0.038) were significantly influenced by the interaction between dominance status and conflict outcome (see table 3 in the electronic supplementary material). The dominant breeding pair increased their donation of body allopreening more than subordinate helpers, but did so especially after losing conflicts (figure 3a). Helpers received a greater increase in body allopreening compared with the breeding pair, but the increase was most apparent following losing conflicts (figure 3b).
Figure 3.
Mean (±s.e.m.) post-conflict changes in body allopreening (a) donation and (b) receipt rate for green woodhoopoe individuals of different dominance status (n=20 dominant breeders (white bars), 16 subordinate helpers (grey bars)) in 10 groups.
4. Discussion
Despite the regular occurrence of intergroup conflicts in many social animal species (McComb et al. 1994; Wilson et al. 2001; Radford 2003; Kitchen & Beehner 2007), their potential impact on intragroup behaviour has received relatively little empirical attention (but, see Cheney 1992; Radford 2008). Here, I demonstrate that intragroup allopreening increases following conflict between neighbouring green woodhoopoe groups, and show for the first time that both the duration and the outcome of these intergroup conflicts influence the amount of affiliative behaviour displayed. Increases in intragroup allopreening were highest following long conflicts and those that were lost, which might explain why Radford (2008) found no increase in intragroup allopreening following the playback of a single rally from a neighbouring group; this would constitute a short, winning conflict for the focal group.
Affiliative behaviour following intragroup conflict often serves to reconcile former opponents (Aureli et al. 2002). Reconciliation cannot explain the increase in allopreening following conflict between green woodhoopoe groups, however, because members of the same group preen one another rather than their rivals. Instead, the affiliative behaviour may be used to reduce the social stress, mediated by glucocorticoid hormones, that is induced by conflict (von Holst 1998); the receipt of allogrooming, the mammalian equivalent of allopreening, is known to decrease an individual's heart rate and tension-related activities (Schino et al. 1998; Aureli et al. 1999). A stress-reduction argument would explain the greater increase in woodhoopoe allopreening following losing intergroup conflicts, because residents are likely to be more stressed if their territory is invaded than if intruders retreat. Likewise, a greater increase in allopreening would be expected following longer intergroup conflicts; not only do individuals have to participate for longer, but conflicts of different length are likely to fulfil different roles and longer conflicts might be more stressful. Whereas short conflicts may simply offer opportunities to assess the composition of neighbouring groups, and thus potential breeding vacancies (Radford 2003), longer conflicts may serve a more aggressive, expansive function, with groups attempting to invade other territories (Radford & du Plessis 2004a).
The increase in post-conflict allopreening of helpers by the breeding pair, especially following losing conflicts, may be an attempt to enhance the former's participation in the future (see also Radford 2008). Allopreening might be used in a similar fashion to allogrooming to strengthen the bonds between the group members (Dunbar 1991), thus increasing the likelihood of individuals assisting in subsequent ventures. Alternatively, owing to its stress-reducing capability, allopreening may be exchanged directly for intergroup conflict participation; allogrooming is often traded for a variety of commodities (Seyfarth & Cheney 1984; de Waal 1997; Barrett et al. 1999). Full participation of group members in intergroup conflicts is important because relative group size often determines their outcome (Radford & du Plessis 2004a). Moreover, although all adult woodhoopoes participate in territorial defence, subordinate helpers expend the most effort (Radford 2003), as is also the case in other species (e.g. Cant et al. 2002); the participation of helpers is therefore crucial from the breeding pair's perspective. Individuals have previously been suggested to groom others in exchange for support in intragroup conflict (see Schino 2007), but my results suggest that affiliative behaviour might also be used to enhance social cohesion and thus maximize future success in intergroup conflicts.
At least partially as a consequence of the post-conflict increase in affiliative behaviour, woodhoopoe groups that were involved in more intergroup conflict had higher overall rates of intragroup allopreening; it is possible that groups that are more heavily involved in intergroup conflict also have higher rates of allopreening at all times. My results therefore lend empirical support to the theoretical idea that intragroup affiliation should be most apparent when intergroup conflict is highest (Hamilton 1975; Alexander & Borgia 1978). Maintenance of intragroup cohesion is likely to be most important when the combined actions of group members are vital (Reeve & Hölldobler 2007), as is the case with woodhoopoe vocal rallying (Radford & du Plessis 2004a). Because up to 10 per cent of green woodhoopoe helpers are unrelated to either of the breeding pair (du Plessis 1993), future studies might consider variation in the level of conflict-related affiliation depending on the specific social and genetic relationships between group members (see Cheney 1992). It is noteworthy, however, that human studies have found a positive relationship between intragroup cooperation and intergroup conflict in groups composed of both kin and non-kin (West et al. 2002, 2006).
My results demonstrate that certain characteristics of an intergroup conflict, namely its duration and outcome, and the individuals involved, namely their dominance status, can influence the level of intragroup affiliative behaviour subsequently displayed. This is important because an increase in, for example, allopreening reduces the time for other vital activities, such as feeding. It remains to be investigated whether characteristics of the groups involved in the conflict, such as their relative sizes or their composition and residency status, also influence the amount of post-conflict affiliative behaviour shown. Moreover, it would be worth examining whether differences between individuals in intergroup conflict participation can help to explain the substantial variation in cooperative activities found between group members in many animal societies (Cockburn 1998). In general, studies examining how interactions between groups affect within-group processes are crucial for our understanding of cooperation and group dynamics.
Acknowledgments
This study complies with the current laws in the country in which it was conducted.
I am grateful to Morné du Plessis for access to the study population he originally established, and to Sarah Hodge, Linda Hollén, Goran Spong, Stu West and two anonymous referees for their comments on the manuscript. The work was supported by a NERC studentship and a BBSRC David Phillips fellowship.
Supplementary Material
Additional methods and results tables
References
- Alexander R.D, Borgia G. Group selection, altruism and the levels of organisation of life. Annu. Rev. Ecol. Syst. 1978;9:449–474. doi:10.1146/annurev.es.09.110178.002313 [Google Scholar]
- Aureli F, Preston S.D, de Waal F.B.M. Heart rate responses to social interactions in free-moving rhesus macaques (Macaca mulatta): a pilot study. J. Comp. Psychol. 1999;113:59–65. doi: 10.1037/0735-7036.113.1.59. doi:10.1037/0735-7036.113.1.59 [DOI] [PubMed] [Google Scholar]
- Aureli F, Cords M, van Schaik C.P. Conflict resolution following aggression in gregarious animals: a predictive framework. Anim. Behav. 2002;64:325–343. doi:10.1006/anbe.2002.3071 [Google Scholar]
- Barrett L, Henzi S.P, Weingrill T, Lycett J.E, Hill R.A. Market forces predict grooming reciprocity in female baboons. Proc. R. Soc. B. 1999;266:665–670. doi:10.1098/rspb.1999.0687 [Google Scholar]
- Cant M.A, Otali E, Mwanguhya F. Fighting and mating between groups in a cooperatively breeding mammal, the banded mongoose. Ethology. 2002;108:541–555. doi:10.1046/j.1439-0310.2002.00795.x [Google Scholar]
- Cheney D.L. Intragroup cohesion and intergroup hostility: the relation between grooming distributions and intergroup competition among female primates. Behav. Ecol. 1992;3:334–345. doi:10.1093/beheco/3.4.334 [Google Scholar]
- Choi J.-K, Bowles S. The coevolution of parochial altruism and war. Science. 2007;318:636–640. doi: 10.1126/science.1144237. doi:10.1126/science.1144237 [DOI] [PubMed] [Google Scholar]
- Cockburn A. Evolution of helping behaviour in cooperatively breeding birds. Annu. Rev. Ecol. Syst. 1998;29:141–177. doi:10.1146/annurev.ecolsys.29.1.141 [Google Scholar]
- de Waal F.B.M. The chimpanzee's service economy: food for grooming. Evol. Human Behav. 1997;18:375–386. doi:10.1016/S1090-5138(97)00085-8 [Google Scholar]
- Dunbar R.I.M. Functional significance of social grooming in primates. Folia Primatol. 1991;57:121–131. [Google Scholar]
- du Plessis M.A. Helping behaviour in cooperatively breeding green woodhoopoes: selected or unselected trait? Behaviour. 1993;127:49–65. doi:10.1163/156853993X00425 [Google Scholar]
- Hamilton W.D. Innate social aptitudes of man: an approach from evolutionary genetics. In: Fox R, editor. Biosocial anthropology. Malaby Press; London, UK: 1975. pp. 133–155. [Google Scholar]
- Heinsohn R, Packer C. Complex cooperative strategies in group-territorial African lions. Science. 1995;269:1260–1262. doi: 10.1126/science.7652573. doi:10.1126/science.7652573 [DOI] [PubMed] [Google Scholar]
- Kitchen D.M, Beehner J.C. Factors affecting individual participation in group-level aggression among non-human primates. Behaviour. 2007;144:1551–1581. doi:10.1163/156853907782512074 [Google Scholar]
- Koski S.E, de Vries H, van den Tweel S.W, Sterck E.H.M. What to do after a fight? The determinants and inter-dependency of post-conflict interactions in chimpanzees. Behaviour. 2007;144:529–555. doi:10.1163/156853907780713082 [Google Scholar]
- Ligon J.D, Ligon S.H. Green woodhoopoes: life-history traits and sociality. In: Slater P.B, Koenig W.C, editors. Cooperative breeding in birds. Cambridge University Press; Cambridge, UK: 1990. pp. 33–65. [Google Scholar]
- McComb K, Packer C, Pusey A. Roaring and numerical assessment in contests between groups of female lions, Panthera leo. Anim. Behav. 1994;47:379–387. doi:10.1006/anbe.1994.1052 [Google Scholar]
- Radford A.N. Territorial vocal rallying in the green woodhoopoe: influence of rival group size and composition. Anim. Behav. 2003;66:1035–1044. doi:10.1006/anbe.2003.2292 [Google Scholar]
- Radford A.N. Vocal mediation of foraging competition in the cooperatively breeding green woodhoopoe, Phoeniculus purpureus. Behav. Ecol. Sociobiol. 2004a;56:279–285. doi:10.1007/s00265-004-0785-6 [Google Scholar]
- Radford A.N. Voice breaking in males results in sexual dimorphism of green woodhoopoe calls. Behaviour. 2004b;141:555–569. doi:10.1163/1568539041166726 [Google Scholar]
- Radford A.N. Type of threat influences postconflict allopreening in a social bird. Curr. Biol. 2008;18:R114–R115. doi: 10.1016/j.cub.2007.12.025. doi:10.1016/j.cub.2007.12.025 [DOI] [PubMed] [Google Scholar]
- Radford A.N, du Plessis M.A. Bill dimorphism and foraging niche partitioning in the green woodhoopoe. J. Anim. Ecol. 2003;72:258–269. doi:10.1046/j.1365-2656.2003.00697.x [Google Scholar]
- Radford A.N, du Plessis M.A. Territorial vocal rallying in the green woodhoopoe: factors affecting contest length and outcome. Anim. Behav. 2004a;68:803–810. doi:10.1016/j.anbehav.2004.01.010 [Google Scholar]
- Radford A.N, du Plessis M.A. Green woodhoopoe territories remain stable despite group-size fluctuations. J. Avian Biol. 2004b;35:262–268. doi:10.1111/j.0908-8857.2004.03235.x [Google Scholar]
- Radford A.N, du Plessis M.A. Dual function of allopreening in the cooperatively breeding green woodhoopoe, Phoeniculus purpureus. Behav. Ecol. Sociobiol. 2006;61:221–230. doi:10.1007/s00265-006-0253-6 [Google Scholar]
- Reeve H.K, Hölldobler B. The emergence of a superorganism through intergroup competition. Proc. Natl Acad. Sci. USA. 2007;104:9736–9740. doi: 10.1073/pnas.0703466104. doi:10.1073/pnas.0703466104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schino G. Grooming and agonistic support: a meta-analysis of primate reciprocal altruism. Behav. Ecol. 2007;18:115–120. doi:10.1093/beheco/arl045 [Google Scholar]
- Schino G, Rosati L, Aureli F. Intragroup variation in conciliatory tendencies in captive Japanese macaques. Behaviour. 1998;135:897–912. [Google Scholar]
- Seyfarth R.M, Cheney D.L. Grooming, alliances, and reciprocal altruism in vervet monkeys. Nature. 1984;308:541–543. doi: 10.1038/308541a0. doi:10.1038/308541a0 [DOI] [PubMed] [Google Scholar]
- von Holst D. The concept of stress and its relevance for animal behaviour. Adv. Study Behav. 1998;27:1–131. doi:10.1016/S0065-3454(08)60362-9 [Google Scholar]
- Watts D.P, Mitani J.C. Boundary patrols and intergroup encounters in wild chimpanzees. Behaviour. 2001;138:299–327. doi:10.1163/15685390152032488 [Google Scholar]
- West S.A, Pen I, Griffin A.S. Cooperation and competition between relatives. Science. 2002;296:72–75. doi: 10.1126/science.1065507. doi:10.1126/science.1065507 [DOI] [PubMed] [Google Scholar]
- West S.A, Gardner A, Shuker D.M, Reynolds T, Burton-Chellow M, Sykes E.M, Guinnee M.A, Griffin A.S. Cooperation and the scale of competition in humans. Curr. Biol. 2006;16:1103–1106. doi: 10.1016/j.cub.2006.03.069. doi:10.1016/j.cub.2006.03.069 [DOI] [PubMed] [Google Scholar]
- Wich S.A, Sterck E.H.M. Familiarity and threat of opponents determine variation in Thomas langur (Presbytis thomasi) male behaviour during between-group encounters. Behaviour. 2007;144:1583–1598. doi:10.1163/156853907782512065 [Google Scholar]
- Wilson M.L, Hauser M.D, Wrangham R.W. Does participation in intergroup conflict depend on numerical assessment, range location, or rank for wild chimpanzees? Anim. Behav. 2001;61:1203–1216. doi:10.1006/anbe.2000.1706 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional methods and results tables