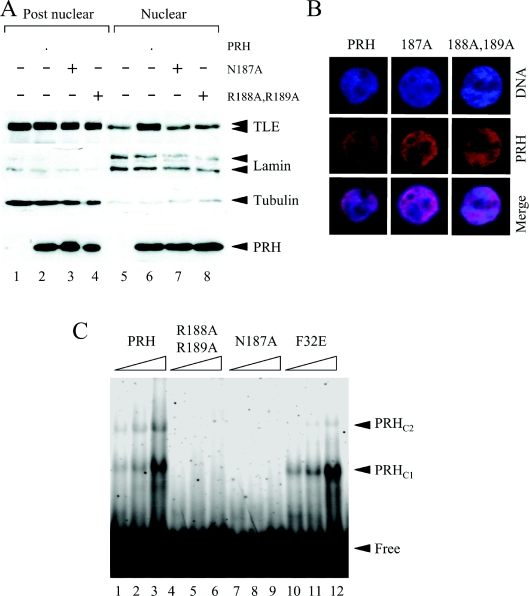
Figure 3. Mutations in the PRH homeodomain block nuclear retention of TLE.
(A) Untransfected K562 cells and cells transiently transfected with vectors expressing Myc–PRH, Myc–PRH N187A or Myc–PRH R188A,R189A were fractionated into post-nuclear and nuclear extracts. The proteins were then separated by SDS/PAGE and Western blotted for endogenous TLE (top panel), lamin A/C (second panel), tubulin (third panel) or Myc–PRH proteins (bottom panel) as described in Figure 1(C). (B) K562 cells growing on coverslips were transiently transfected with vectors expressing Myc–PRH, Myc–PRH N187A or Myc–PRH R188A,R189A. DNA was stained with DAPI (blue) and Myc–PRH was visualized using an anti-Myc9E10 antibody and a TRITC-labelled secondary antibody (red). The cells were viewed using a Leica DM IRBE confocal microscope. (C) A labelled oligonucleotide carrying a PRH-binding site was incubated with increasing concentrations (125, 250 and 500 nM) of His-tagged PRH (lanes 1–3), His-tagged PRH R188A,R189A (lanes 4–6), PRH N187A (lanes 7–9) and PRH F32E (lanes 10–12) under the conditions described in the main text. Free and bound DNA was then resolved on a 6% polyacrylamide gel and visualized using a PhosphoImager. Two retarded complexes (PRHC1 and PRHC2) are formed when PRH binds to the DNA.