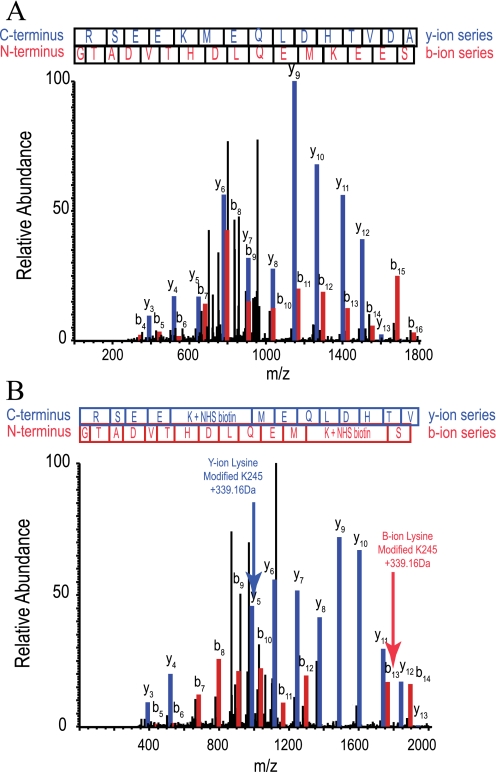
FIGURE 7.
MS/MS spectra of a GLUT1 peptide modified by sulfo-NHS-LC-biotin. Sulfo-NHS-LC-biotin covalently modifies lysine side chain primary amines producing a covalent mass addition of 339.16 Da. A, MS/MS spectrum of a GLUT1 tryptic peptide R232↓GTADVTHDLQEMKEESR249↓Q from L6–7. The peptide elutes at 10.47 min (26% organic solvent). The y- and b-ion series (blue and red, respectively) confirm the identity of the unmodified peptide with Sequest scoring parameters as follows: XCorr, 4.636; peptide probability, 3.4 × 10-9; ΔCn, 0.740; and preliminary score, 1975.5. B, the same peptide, except modified at Lys245 by sulfo-NHS-LC-biotin. Modification is first observed at y5 and b13, which show a 339.16-Da lysine adduct. All sequential ions in each series show this mass adduct. The modified peptide elutes at 19.64 min (31% organic solvent). The MS/MS spectrum and Sequest scoring parameters confirm its identity (XCorr, 4.652; peptide probability, 5 × 10-9; ΔCn, 0.615; and preliminary score, 1235.2