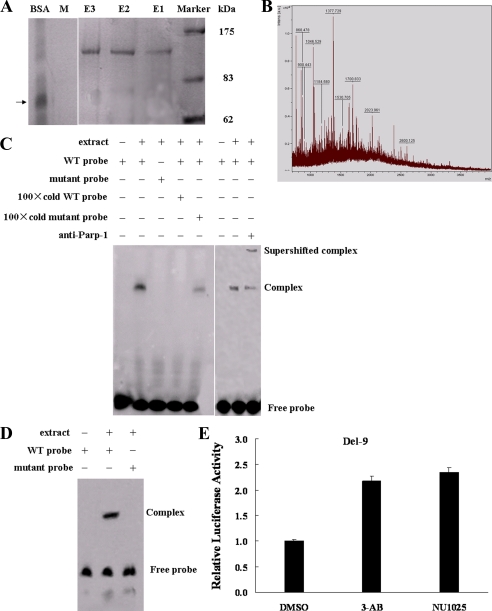
FIGURE 2.
Parp-1 binds to the BRCA2 promoter. A, MCF-7 cell nuclear extracts were mixed with a biotinylated WT probe and a mutant probe (M). The DNA-protein complex was isolated with streptavidin-labeled magnetic beads. The magnetic bead column was washed and eluates were collected. Eluted fractions were separated on 10% acrylamide gels and visualized by silver staining. The 120-kDa protein band indicates a prominent DNA-protein complex. BSA was used as a control, and its position is indicated by a black arrow. The experiment was carried out three times. E1, E2, E3 (WT probe eluates); M, mutant probe eluate. B, mass spectrometry of the ∼120-kDa protein. C, EMSAs and antibody super-shifts were performed in MCF-7 nuclear extracts using WT, mutant probes, and Parp-1 antibody. D, EMSAs were carried out in MCF-7 nuclear extracts using the end-streptavidin blocked-biotin-labeled WT and mutant probes.E, luciferase activity of Del-9 in MCF-7 cells treated with 3-AB/NU1025, two Parp-1 inhibitors, or DMSO for 12 h.