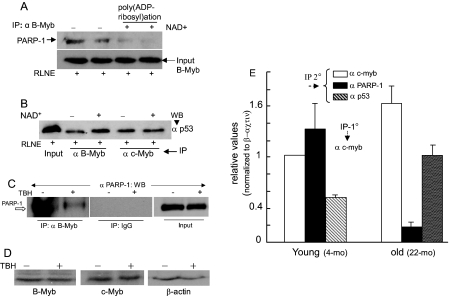
FIGURE 8.
Effects of β-NAD+, oxidative stress, and aging on Myb interaction with PARP-1 and p53. RLNE was incubated at 37 °C with β-NAD+ (500 μm)-containing buffer to promote PARP-1-mediated poly(ADP-ribosyl)ation and then immunoprecipitated with B-Myb antibody (A) and B-Myb and c-Myb antibodies (B) followed by Western blotting as shown. Duplicate lanes in A correspond to duplicate experiments. C, B-Myb/PARP-1 interaction in H4IIE cells in the presence or absence of TBH (4 μm). D, Western blot assay of B-Myb, c-Myb, andβ-actin levels in H4IIE cells that were treated with vehicle or TBH. 50 μg of protein was used per lane. E, sequential ChIP (first ChIP with anti-c-Myb) on liver chromatin of young (4 months) and old rats (22 months). Two rats from each group were analyzed separately, and q-PCR was run in triplicate. Combined data from each age is presented as the average ± S.D.