FIGURE 1. Affinity capture of mitochondrial ribosomal protein L12 (MRPL12) in association with POLRMT, the human mitochondrial RNA polymerase.
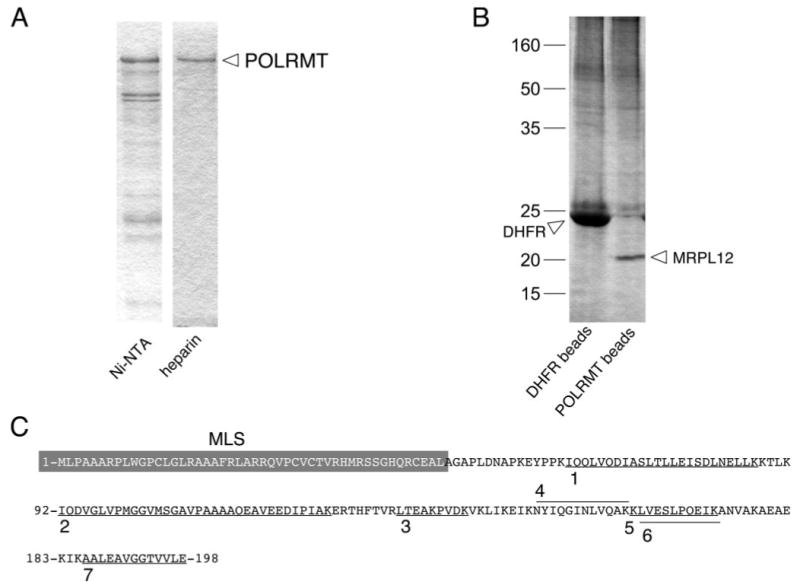
A, purification of His-tagged POLRMT by nickel-affinity and heparin-agarose chromatography. Shown are Coomassie-stained SDS-PAGE gels of representative fractions from a typical purification of recombinant POLRMT from E. coli lysate after nickel-affinity chromatography (Ni-NTA) and subsequent heparin-agarose (heparin) chromatography. The ∼130-kDa POLRMT band is indicated. B, results of affinity capture of POLRMT-binding proteins from HeLa cell mitochondrial lysates. Shown is a silver-stained SDS-PAGE gel of proteins eluted from control DHFR beads (left lane) or POLRMT beads (right lane). The major ∼20-kDa band that co-purified with POLRMT is labeled MRPL12, based on the mass spectroscopy-based identification performed on the excised band. C, mass spectroscopy-based peptide analysis identified the 20-kDa band in panel B as MRPL12. The seven peptides unambiguously identified as MRPL12 are underlined (or overlined) on the primary sequence of the human MRPL12 polypetide (amino acids 1–198) and labeled 1–7. The gray box indicates the mitochondrial localization sequence (MLS) of MRPL12. Note that no peptides were identified in this region as would be predicted if the MLS were cleaved off upon import.
