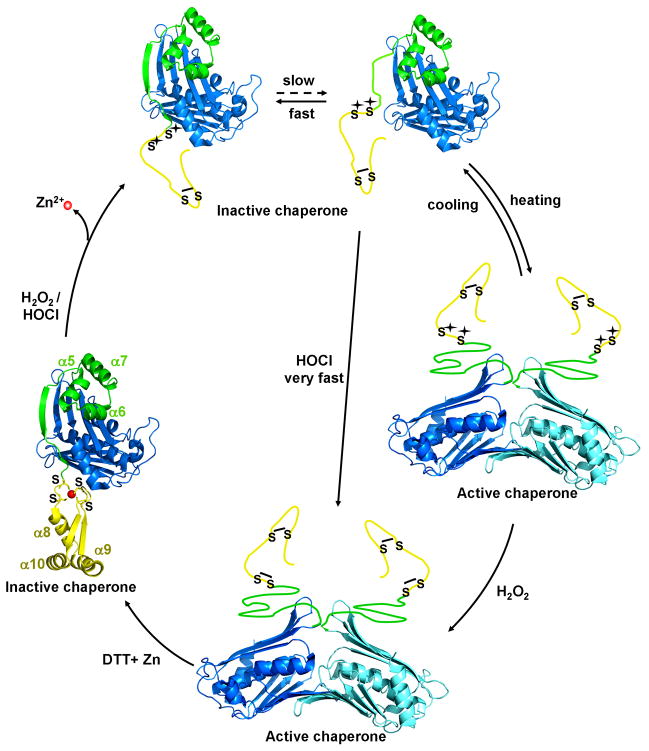
Fig. 7. Hsp33 - A paradigm for HOCl’s oxidative unfolding activity.
Hsp33 consists of an N-terminal domain (blue), a redox sensitive zinc binding domain (yellow) and a flexible linker region (green), which serves as folding sensor in Hsp33. Upon oxidative modification of Hsp33’s distal cysteines (Cys265/Cys268), zinc is released and Hsp33’s zinc binding domain unfolds. This destabilizes the linker region, which is now in a dynamic equilibrium between the folded state, in which no thiol oxidation can occur and an unfolded state, in which oxidative modification can occur with a rate constant determined by the oxidant. Kinetically slow oxidants, such as H2O2 require mild unfolding conditions (e.g., heat) to react with the critical cysteine pair Cys232/Cys234. Kinetically fast oxidants such as HOCl, however, compete with the refolding reaction and allow oxidation even in the absence of unfolding conditions. Disulfide bond formation prevents refolding of Hsp33’s redox switch domain and converts Hsp33 into highly active molecular chaperone.