Abstract
BACKGROUND
In previous studies, we found that approximately 25% of patients having carotid endarterectomy with general anesthesia (CEA general) develop cognitive dysfunction compared with a surgical control Group 1 day and 1 mo after surgery. In this study, we tested the hypothesis that patients having CEA with regional anesthesia (CEA regional) will develop significant cognitive dysfunction 1 day after surgery compared with a control group of patients receiving sedation 1 day after surgery. We did not study persistence of dysfunction.
METHODS
To test this hypothesis, we enrolled 60 patients in a prospective study. CEA regional was performed with superficial and deep cervical plexus blocks in 41 patients. The control group consisted of 19 patients having coronary angiography or coronary artery stenting performed with sedation. A control group is necessary to account for the “practice effect” associated with repeated cognitive testing. The patients from the CEA regional group were enrolled at New York Medical Center and the control group at Columbia-Presbyterian Medical Center. The cognitive performance of all patients was evaluated using a previously validated battery of neuropsychometric tests. Differences in performance, 1 day after compared with before surgery, were evaluated by both event-rate and group-rate analyses.
RESULTS
On postoperative day 1, 24.4% of patients undergoing CEA regional had significant cognitive dysfunction, where “significant” was defined as a total deficit score ≥2 SD worse than the mean performance in the control group.
CONCLUSIONS
Patients undergoing CEA regional had an incidence of cognitive dysfunction which was not different than patients having CEA general as previously published and compared with a contemporaneously enrolled group.
Carotid endarterectomy (CEA) reduces the risk of future stroke in patients with high-grade stenosis.1–5 Although the incidence of new neurologic findings associated with CEA is between 3% and 5%,5 approximately 25% of CEA patients having general anesthesia (CEA general) develop significant postoperative neurocognitive dysfunction based on performance on a battery of neuropsychometric tests administered before and after surgery when examined 1 day and 1 mo after surgery.6,7 We chose to define “significant” dysfunction as cognitive performance that is two standard deviations (SD) worse than performance in an appropriate control group. Although the mechanism of postoperative neurocognitive dysfunction is poorly understood, it is believed to be ischemic in nature, likely due to hypoperfusion during carotid artery cross-clamping. However, general anesthesia itself may contribute significantly to cognitive dysfunction.8–11
We hypothesized that patients scheduled for elective CEA with regional anesthesia (CEA regional) would develop short-term cognitive dysfunction at 1 day after surgery compared with a control group of patients if cognitive dysfunction was due to surgery. Cognitive performance in patients having CEA regional was compared with performance in a control group having coronary artery angiography and/or stenting (control) to account for the “practice effect” associated with repeated testing, and for the effect of mild sedation.
METHODS
Study Population
Sixty patients were prospectively enrolled in this IRB-approved study, of whom 41 were scheduled for elective CEA received regional anesthesia (enrollment March 2004 to January 2007). All CEA patients had 60% or more stenosis of the operative carotid artery. We enrolled a control group to account for the practice effect. The control group consisted of 19 patients (enrollment November 2005 to February 2007) undergoing coronary artery stenting and/or coronary angiography with sedation. None of these patients had been reported in any previous studies.
After obtaining written informed consent, patients were evaluated with a battery of six neuropsychometric tests before surgery and 1 day after the procedure.
Anesthesia and Surgery
CEA regionals were performed at New York University Medical Center (NYUMC). All patients received supplemental oxygen at 5 L/min via facemask. Noninvasive monitors included electrocardiogram, arterial blood pressure, and pulse oximeter. Because of the frequent incidence of subclavian stenosis in CEA patients, the arterial line was placed in the arm with the higher noninvasive pressure to obtain a more accurate reading of arterial blood pressure or, if equal, then in the ipsilateral arm under local anesthesia. The reason for this configuration was that when the carotid was clamped, patients needed to squeeze a toy, as a measure of strength, with the contralateral hand. The arterial catheter made this task difficult.
CEA regional patients underwent superficial and deep cervical plexus blocks using mepivacaine 1.5% with epinephrine 1:200,000. Details of this technique are given elsewhere.12 All patients were sedated with dexmedetomidine and varying doses of fentanyl and/or midazolam. Upon clamping the carotid artery, all patients were evaluated for speech deficit (counting), contralateral arm strength (ability to squeeze a toy), and significant increase in agitation or confusion. Patients who demonstrated signs of cerebral ischemia with test clamping were treated with intraarterial shunting. Patients recovered in a postoperative care unit.
Coronary artery stenting were performed at Columbia University Medical Center. The patients undergoing coronary artery stenting or coronary angiography received an injection of local anesthetic, lidocaine 2%, in the area of the femoral puncture. They were sedated with fentanyl and/or midazolam. One patient received 2 mg of morphine.
Surgery
CEAs were performed by members of the vascular service at NYUMC (mainly by T.S.R., but a few by T.M.). The surgery for CEA consisted of positioning the patient supine with the head in an extended midline position. An incision was made along a skin crease from just below the angle of the mandible to near the midline through skin, subcutaneous tissue, and platysma. The common, internal, and external carotid arteries were exposed and controlled. All CEA patients received a heparin bolus prior to clamping the carotid artery. A dacron Hemashield© patch was used in all patients and protamine reversal was given at the end of surgery.
Neuropsychometric Evaluation
Patients were assessed with a battery of six neuropsychometric tests, chosen to represent a range of cognitive domains, before and 1 day (approximately 20 h) after surgery. Neuropsychometric tests were administered by one of three research assistants who were trained at the Columbia University site by a senior neuropsychologist and went to NYU to recruit and administer all of the tests. Before and after surgery, evaluations were performed by the same research assistant. The Boston Naming Test evaluated patients’ ability to verbally identify objects pictured on a series of cards. Halstead-Reitan Trails Parts A (Trails A) and B (Trails B) evaluated visual conceptual and visuomotor tracking by timing how long it took a subject to connect consecutively numbered circles with a single line (part A) and then connect the same number of consecutively numbered and lettered circles by alternating between the two sequences (part B). The Controlled Oral Word Association test assessed verbal fluency, providing information on dominant hemisphere function. Patients were asked to generate as many words as possible that begin with a certain letter within 60 sec. Three separate trials were performed at each testing session, one each with the letters C, F, and L. Scoring for these tests is described in more detail elsewhere.13 The copy portion of the Rey Complex Figure test (Rey Copy) evaluated visuo-spatial organization, providing insight into the functioning of the nondominant hemisphere. Patients were asked to copy a complex figure and a standardized scoring system was used to evaluate the presence of design-specific features and the accuracy of their locations.14 Afterwards, they were asked to draw the same figure from memory (Rey Recall).14
Statistical Analysis
Perioperative variables and neurocognitive performance for all patient groups, CEA regional and control patients, were calculated and compared using Fisher’s exact test, or the nonparametric Wilcoxon’s ranked SUM test using SAS™ version 8.2 software (SAS Institute, Cary, NC).
Change in cognitive performance was analyzed by using the performance of patients in the control group to determine the mean and SD of change scores between pre- and postoperative performance on each of the neuropsychometric tests. Z-scores for performance were calculated for patients in both CEA groups.
controls where Δ refers to change in neuropsychometric performance either of patients having CEA or control patients, and SD is the standard deviation of the change scores of the patients in the control group. Z-scores were converted to a point deficit score by assigning points from “0” to “5” in −0.5 Z-score increments, as described previously.7 For each patient, these deficit points were summed to generate a total deficit score that measures the global level of cognitive decline of each patient at day 1. By convention for abnormality, we defined significant neurocognitive dysfunction as a total deficit score exceeding the mean total deficit score of the control group by two SDs. Performance was also analyzed using continuous data. For continuous data, Wilcoxon’s ranked sum test were used to test group differences in neuropsychometric performance using both Z-scores that reflect both improvements and declines in cognitive performance and point deficit scores that measure only declines in cognitive performance. A Bonferroni adjustment was made to the P values obtained for individual tests.
RESULTS
Cohort Characteristics
The perioperative variables for the two groups of patients, CEA regional and controls, are presented in Table 1. There were no significant differences between the two groups except the control patients had fewer transient ischemic attacks and strokes by history (27% vs 5%). Seventy-three percent of patients having CEA regional were asymptomatic. Intraoperative values are shown in Table 2. The duration of the control procedures were significantly shorter than CEA surgery, and the medications administered are less. Patients in the CEA regional group received dexmedetomidine, which was not administered to patients in the control group. Mean duration of surgery and duration of carotid artery cross-clamp were 117.3 ± 33.3 minutes, and 55.0 ± 12.2 minutes, respectively, where “± ” is the SD (Table 2). The percentage of patients requiring a shunt was 2.5% for CEA regional (Table 2).
Table 1.
Perioperative Data
| CEA regional | Control | |
|---|---|---|
| Number of patients | 41 | 19 |
| Age (yrs) | 70.9 (10.5) | 63.5 (11.0) |
| Gender (male/female) | 78.0%/22.0% | 63.2%/36.8% |
| Handedness (right/left) | 90.2%/9.8% | 84.2%/15.8% |
| Height (cm) | 172.6 (8.6) | 168.4 (11.4) |
| Weight (kg) | 80.1 (13.7) | 85.5 (22.3) |
| Education (yrs) | 16.4 (3.3) | 15.6 (4.5) |
| Smoker (%) (pk yrs) | 82.9% (38.6) | 68.4% (37.8) |
| Hypertension (%) | 66 | 79 |
| Diabetes mellitus (%) | 22 | 21 |
| Previous stroke or TIA (%)a | 27 | 5 |
| Previous MI (%) | 22 | 26 |
Mean and standard deviation are given in parenthesis.
CEA = carotid endarterectomy; TIA = transient ischemic attack; MI = myocardial infarction; CEA regional = patients having CEA under a regional anesthetic; Controls = patients having coronary artery angioplasty and/or stenting under local sedation.
Statistically different from Controls having artery stenting at P < 0.05.
Table 2.
Intraoperative Data
| Intraoperative | CEA regional | Control |
|---|---|---|
| Duration of surgery (min) | 117.3 (33.3) | 73.2 (30.6) |
| Duration of cross-clamp (min) | 55.0 (12.2) | – |
| Fentanyl (mcg/kg) | 1.3 (0.8) | 0.8 (0.06) |
| Midazolam (mg/kg) | 0.03 (0.02) | 0.02 (0.01) |
| Dexmedetomidine (mcg/kg/surgery) | 43.7 (19.6) | – |
| Heparin (units) | 6,043.5 (1,444.6) | – |
| Protamine (mg) | 18.2 (8.3) | – |
| Shunt (%age) | 2.5% | – |
Values refer to mean. Numbers in parenthesis represent the standard deviation.
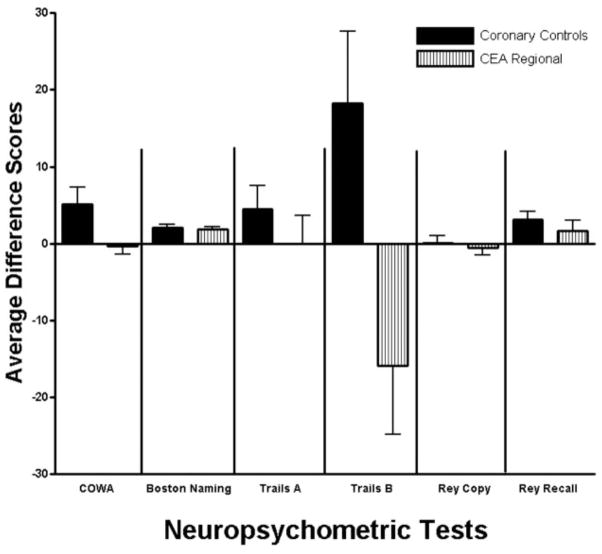
Baseline preoperative neuropsychometric scores for each of the tests are shown in Table 3. There were no significant differences in preoperative cognitive performance between the two groups. Average difference scores before versus after surgery or coronary artery angiography and/or stenting are depicted for each test for each of the patient groups (Fig. 1). Because pain is a significant confounder for evaluation of neuropsychometric performance, visual analog pain scale scores were determined at each examination (Table 4). Although there was a significant increase in pain after surgery compared with before (Baseline) for the CEA regional group, and between groups (except for pain standing 1 day after surgery), no patient had visual analog pain scale scores ≥4, which we have previously demonstrated to interfere with cognitive performance (Table 4). Average cognitive performance improved for patients in the control group except for patient performance on the Rey Copy test, where it was unchanged. There were significant differences between the two groups in performance on Trails B (Fig. 1).
Table 3.
Baseline Scores
| CEA regional | Control | |
|---|---|---|
| COWA | 45.0 (13.3) | 39.8 (12.9) |
| Boston naming | 55.4 (4.7) | 51.2 (8.8) |
| Trails A (sec) | 44.8 (16.3) | 45.3 (18.5) |
| Trails B (sec) | 103.3 (56.5) | 115.8 (78.8) |
| Rey Copy | 26.9 (6.3) | 26.7 (5.8) |
| Rey Recall | 13.6 (10.2) | 12.7 (7.6) |
Mean and standard deviation are given in parenthesis. Scoring for these tests is described in detail in Ref. 14.
COWA = controlled oral word association test; Rey Copy = the Rey Complex Figure test, copy component; Rey Recall = the Rey Complex Figure test, recall component.
Figure 1.
Change scores by test. The average difference scores for these six tests are generated by subtracting the performance score “after” from “before” surgery or the coronary procedure for all tests except Trails A and B, where just the reverse subtraction is performed. In this way, positive values indicate improved performance. The error bars represent the standard error of the mean (S.E.M.). The two groups are represented by this bar graph: solid black bars, the control group; and vertical striped, carotid endarterectomy (CEA)-regional group.
Table 4.
Visual Analog Pain Scores
| Pain: sitting
|
Pain: standing
|
|||||
|---|---|---|---|---|---|---|
| Group | Baseline | 1 day | T-Testa | Baseline | 1 day | T-Testa |
| CEA regional | 0.3 ± 0.9 | 1.9 ± 2.1 | <0.001 | 0.4 ± 0.9 | 1.5 ± 1.9 | 0.001 |
| Control | 1.1 ± 1.1 | 0.7 ± 0.9 | 0.226 | 1.3 ± 1.8 | 1.1 ± 1.4 | 0.770 |
| T-testb | 0.003 | 0.020 | 0.011 | 0.502 | ||
Mean = standard deviation.
T-test comparing baseline pain score to pain score 1 day after surgery or a procedure.
T-test comparing pain score in the two groups of patients: CEA regional to controls at baseline or 1 day after surgery or a procedure.
Individual total deficit scores for each patient in each of the two groups are shown in Figure 2. The mean group performance of patients in the control group was significantly different than group performance in the CEA regional (P = 0.0002) group. By categorical analysis, 10 patients in the CEA regional group (24.4%) had total deficit scores that were ≥2 SD above the mean of the total deficit score of the control group. To verify that change in performance on Trails B did not account for our results regarding the patients with significant cognitive dysfunction based on the total deficit score, we reanalyzed the total deficit score excluding the change in performance on Trails B. Under these conditions, 8 patients had total deficit scores ≥2 SD above the mean of the total deficit score of the control group for an incidence of 19.5%.
Figure 2.
Comparison between groups. The mean total deficit scores are shown by the dashed line for each of the two groups. The mean of the carotid endarterectomy (CEA) regional group is significantly different from the mean of the control group (P = 0.0002). The solid line represents two standard deviations above the mean of the control group. The triangles represent total deficit scores for patients with some triangles representing more than one patient.
DISCUSSION
Although CEA is considered a safe procedure, with a low incidence of perioperative stroke, the prevalence of subtle neurocognitive dysfunction after CEA is being increasingly recognized.7,15,16 Cognitive dysfunction after CEA is detected by a battery of neuropsychometric tests designed to offer a more detailed assessment of higher cortical function than traditional neurological examination. Previously, we demonstrated that approximately 25% of CEA general patients experience a decline in neurocognitive performance on a battery of neuropsychometric tests administered 1 day postoperatively, in comparison to a similarly aged population exposed to a comparable anesthetic regimen.6,7 However, the question remained whether cognitive dysfunction in this population was due in part to the anesthetic technique.
A control group was studied to determine what component of change in performance before and after surgery for each test was due to repeated testing (practice effect). Using the control group, we calculated the mean and SD of the difference scores to calculate Z-scores for each test. With these Z-scores, we summarized the overall cognitive performance for each patient by summing the Z-scores for each test. In this way, performance in each test, and its representative cognitive domain, was weighted without the statistical bias associated with how scores were calculated for each test. The other reason to sum the Z-scores over the total test battery was to provide a cutoff for statistically significant dysfunction. There is a bias to simplify outcome using categorical measures of significant dysfunction.
If cognitive dysfunction were present after CEA Regional, it would add further support to the idea that surgery was the cause; and if cognitive dysfunction were absent or significantly reduced, that general anesthesia was the most likely cause. Since we had previously demonstrated cognitive dysfunction after general anesthesia as being due to surgery at both 1 day and 1 mo after surgery, we chose to look for immediate or short-term effects because we thought that cognitive changes would be seen most easily at this time-point. However, later follow-up times would have given a better idea of the persistence of cognitive dysfunction.
Early manifestation of neurologic signs and cognitive dysfunction still indicate ischemic changes in the brain. Restoration of function may arise because of compensatory cerebral mechanisms that can be quite unique. For example, patients with impaired cerebral blood flow in one hemisphere can recruit different cerebral areas to compensate for the loss of function that may even include the opposite hemisphere.17,18 As well, Newman et al. demonstrated that early cognitive changes in patients having cardiac surgery that resolve considerably by 6 wk and 6 mo after surgery have long-term sequelae in predicting cognitive deterioration 5 years later.19 However, the etiology of the cognitive changes may determine the course of cognitive performance. For patients not having cardiac or carotid artery surgeries, the effect of anesthesia and surgery decreases over time.8,11,20–22
It has been difficult to find evidence to demonstrate a difference between regional and general anesthesia, even in studies enrolling large numbers of patients. Two large studies of patients having noncardiac and noncarotid artery surgery used neuropsychometric tests to determine whether regional or general anesthesia reduced postoperative cognitive dysfunction.23,24 Williams-Russo et al.23 were unable to find a benefit associated with regional anesthesia; however, while Rasmussen et al. were unable to show an advantage if they analyzed their data based on intention-to-treat, they did show a significant advantage if they excluded surgical cases which did not receive the allocated anesthetic.24
Previous studies have compared regional versus general anesthesia in patients having CEA using the presence of new neurologic findings as the primary end-point. Because the incidence of new neurologic findings is on the order of 3% to 5%, a large number of patients have to be enrolled to have statistical power to demonstrate a difference in these two anesthetic techniques. Guay was able, only by meta-analysis of retrospective combined with prospective studies, to suggest that outcome improved with regional anesthesia.25
In addition, we enrolled a contemporaneous group of 69 patients having CEA general to demonstrate that our current neuropsychometric techniques lead to a similar incidence of significant cognitive dysfunction (Appendix online at www.anesthesia-analgesia.org). Using the same criteria to analyze performance as we describe in this article, we found that the incidence of cognitive dysfunction in CEA general was 24.6%. The difference between CEA regional and CEA general was not statistically different (P = 0.82, Fisher’s exact test). However, to have had the statistical power to detect a 10% difference with β equal to 0.80 and α equal to 0.05, one would need about 400 patients per group. Based on our sample size, we could not conclude that these two groups of CEA patients were not significantly different in their incidence of cognitive dysfunction.
There are a few concerns associated with our study. First, total deficit scores were based on Z-scores using patient performance from the control group. Ideally, the control group should have consisted of patients with carotid artery stenosis having anesthesia without surgery, but that was an unobtainable population to study. Patients having coronary artery stenting were not a perfect control group, especially because they may have distal embolization into the carotid circulation most probably from aortic debris.26 However, stroke associated with emboli in this population is small,27 on the order of 0.2%. However, a larger number of patients, 5.9%28 to 15%,29 have evidence for asymptomatic embolic cerebral infarction based on magnetic resonance imaging. These emboli impair cognitive performance after cardiac catheterization.30 If anything, this control group would have made it even more difficult to demonstrate significant cognitive dysfunction, because while we may have seen less cognitive improvement due to the practice effect in patients associated with emboli, the SD of the change scores should have increased. Since the Z-scores in CEA patients are based on the mean and SD of the change scores in the control group, the Z-scores in the CEA group decrease with an increase in the SD of the change scores in the control group.
Second, patients in the control group were sedated with small doses of fentanyl (one patient had morphine) and midazolam (Table 2). However, the CEA regional patients were given dexmedetomidine (Table 2). Dexmedetomidine is an α-agonist used in anesthesia for sedation. As a sedative-hypnotic, its advantage is that it does not suppress respiration, maintains stable hemodynamics and rapidly redistributes with a half-life of 5 min.31 Although we (E.J.H.) have found that prolonged infusions of dexmedetomidine may impair cognitive performance during cerebral endovascular procedures for at least 45 min,32 others at NYUMC have shown that it is an effective adjuvant for sedation during CEA regional.33–35 In addition, we would not expect dexmedetomidine to have any effect on the postoperative evaluation of performance because our patients were examined >20 h after dexmedetomidine was discontinued, which is at least four half-lives beyond the clearance half-life of dexmedetomidine (2 to 5 h36).
The results of this study are identical to our previously reported results7 and that of a contemporaneous study of CEA general patients (not published), and support the view that cognitive dysfunction in the short-term arises from the effect of surgery when the carotid artery is clamped. The precise mechanism of post-CEA neurocognitive dysfunction is unknown, although evidence points to an ischemic etiology. Declines in cognitive function after CEA have been associated with serum elevations of protein S100b, a marker of glial cell death, indicating the occurrence of cerebral injury.37 Ischemia may be due to transient hypoperfusion during carotid cross-clamping or the dislodgement of microemboli. However, in CEA general patients we have been unable to find Doppler HITS (high intensity transients) thought to be associated with microemboli, or focal ischemia on postoperative diffusion-weighted magnetic resonance imaging.38
We found that CEA regional patients have an incidence of cognitive dysfunction similar to that of CEA general patients based on an event-rate analysis, as previously published6,7 and on a contemporaneously enrolled CEA general group (presented in an Appendix online at www.anesthesia-analgesia.org). It is likely that events occurring during surgery are the cause of these cognitive findings, independent of the type of anesthetic technique or cerebral monitoring used.
Acknowledgments
Supported, in part, by NIA RO1 AG17604 to Dr Eric J. Heyer.
Footnotes
This article has supplementary material on the Web site: www.anesthesia-analgesia.org.
References
- 1.North Am Symptomatic Carotid Endarterectomy Trial Collaborative (NASCET) Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. NEJM. 1991;325:445–53. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 2.Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273:1421–8. [PubMed] [Google Scholar]
- 3.Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J, Thomas D. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004;363:1491–502. doi: 10.1016/S0140-6736(04)16146-1. [DOI] [PubMed] [Google Scholar]
- 4.Hobson RW, II, Weiss DG, Fields WS, Goldstone J, Moore WS, Towne JB, Wright CB Group TVACS. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. NEJM. 1993;328:221–7. doi: 10.1056/NEJM199301283280401. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson GG, Eliasziw M, Barr HW, Clagett GP, Barnes RW, Wallace MC, Taylor DW, Haynes RB, Finan JW, Hachinski VC, Barnett HJ. The North Am Symptomatic Carotid Endarterectomy Trial: surgical results in 1415 patients. Stroke. 1999;30:1751–8. doi: 10.1161/01.str.30.9.1751. [DOI] [PubMed] [Google Scholar]
- 6.Heyer E, Adams D, Todd G, Solomon R, Quest D, Steneck S, Connolly E. Neuropsychometric changes in patients after carotid endarterectomy. Stroke. 1998;29:1110–5. doi: 10.1161/01.str.29.6.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heyer EJ, Sharma R, Rampersad A, Winfree CJ, Mack WJ, Solomon RA, Todd GJ, McCormick PC, McMurtry JG, Quest DO, Stern Y, Lazar RM, Connolly ES. A controlled prospective study of neuropsychological dysfunction following carotid end-arterectomy. Arch Neurol. 2002;59:217–22. doi: 10.1001/archneur.59.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abildstrom H, Rasmussen LS, Rentowl P, Hanning CD, Rasmussen H, Kristensen PA, Moller JT. Cognitive dysfunction 1–2 years after non-cardiac surgery in the elderly. ISPOCD group. International Study of Post-Operative Cognitive Dysfunction. Acta Anaesthesiol Scand. 2000;44:1246–51. doi: 10.1034/j.1399-6576.2000.441010.x. [DOI] [PubMed] [Google Scholar]
- 9.Rasmussen LS, Steentoft A, Rasmussen H, Kristensen PA, Moller JT. Benzodiazepines and postoperative cognitive dysfunction in the elderly. ISPOCD Group. International Study of Postoperative Cognitive Dysfunction. Br J Anaesth. 1999;83:585–9. doi: 10.1093/bja/83.4.585. [DOI] [PubMed] [Google Scholar]
- 10.Moller JT. Postoperative cognitive decline: the extent of the problem. Eur J Anaesthesiol. 1998;15:765–7. doi: 10.1097/00003643-199811000-00027. [DOI] [PubMed] [Google Scholar]
- 11.Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Siverstein JH, Beneken JEW, Gravenstein JS, Investigators I. Long-term postoperative cognitive dysfunction in the elderly: ISPOCD1 study. The Lancet. 1998;351:857–61. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 12.Riles T, Gold M. Alternatives to general anesthesia for carotid endarterectomy. In: Moore WS, editor. Surgery for cerebrovascular disease. 2. Philadelphia: WB Saunders; 1996. pp. 338–41. [Google Scholar]
- 13.Spreen O, Strauss E. A compendium of neuropsychological tests: administration, norms, and commentary. 2. New York, NY: Oxford University Press; 1998. [Google Scholar]
- 14.Lu PH, Boone KB, Cozolino L, Mitchell C. Effectiveness of the Rey-Osterrieth Complex Figure Test and the Meyers and Meyers recognition trial in the detection of suspect effort. Clin Neuropsychol. 2003;17:426–40. doi: 10.1076/clin.17.3.426.18083. [DOI] [PubMed] [Google Scholar]
- 15.Vanninen E, Vanninen R, Aikia M, Tulla H, Kononen M, Koivisto K, Partanen J, Partanen K, Hippelainen M, Kuikka JT. Frequency of carotid endarterectomy-related subclinical cerebral complications. Cerebrovasc Dis. 1996;6:272–80. [Google Scholar]
- 16.Vanninen R, Koivisto K, Tulla H, Manninen H, Partanen K. Hemodynamic effects of carotid endarterectomy by magnetic resonance flow quantification. Stroke. 1995;26:84–9. doi: 10.1161/01.str.26.1.84. [DOI] [PubMed] [Google Scholar]
- 17.Krakauer JW, Radoeva PD, Zarahn E, Wydra J, Lazar RM, Hirsch J, Marshall RS. Hypoperfusion without stroke alters motor activation in the opposite hemisphere. Ann Neurol. 2004;56:796–802. doi: 10.1002/ana.20286. [DOI] [PubMed] [Google Scholar]
- 18.Marshall RS, Krakauer JW, Matejovsky T, Zarahn E, Barnes A, Lazar RM, Hirsch J. Hemodynamic impairment as a stimulus for functional brain reorganization. J Cereb Blood Flow Metab. 2006;26:1256–62. doi: 10.1038/sj.jcbfm.9600274. [DOI] [PubMed] [Google Scholar]
- 19.Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, Jones RH, Mark DB, Reves JG, Blumenthal JA. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344:395–402. doi: 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- 20.Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, Gravenstein JS. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 21.Price CC, Garvan CW, Monk TG. Type and severity of cognitive decline in older adults after noncardiac surgery. Anesthesiology. 2008;108:8–17. doi: 10.1097/01.anes.0000296072.02527.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson T, Monk T, Rasmussen LS, Abildstrom H, Houx P, Korttila K, Kuipers HM, Hanning CD, Siersma VD, Kristensen D, Canet J, Ibanaz MT, Moller JT. Postoperative cognitive dysfunction in middle-aged patients. Anesthesiology. 2002;96:1351–7. doi: 10.1097/00000542-200206000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Williams-Russo P, Sharrock NE, Mattis S, Szatrowski TP, Charlson ME. Cognitive effects after epidural vs general anesthesia in older adults. A randomized trial [see comments] JAMA. 1995;274:44–50. [PubMed] [Google Scholar]
- 24.Rasmussen LS, Johnson T, Kuipers HM, Kristensen D, Siersma VD, Vila P, Jolles J, Papaioannou A, Abildstrom H, Silverstein JH, Bonal JA, Raeder J, Nielsen IK, Korttila K, Munoz L, Dodds C, Hanning CD, Moller JT. Does anaesthesia cause postoperative cognitive dysfunction? A randomised study of regional versus general anaesthesia in 438 elderly patients. Acta Anaesthesiol Scand. 2003;47:260–6. doi: 10.1034/j.1399-6576.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 25.Guay J. Regional or general anesthesia for carotid endarterectomy? evidence from published prospective and retrospective studies. J Cardiothorac Vasc Anesth. 2007;21:127–32. doi: 10.1053/j.jvca.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 26.Keeley E, Grines C. Scraping of aortic debris by coronary guiding catheters: a prospective evaluation of 1,000 cases. J Am Coll Cardiol. 1998;32:1861–5. doi: 10.1016/s0735-1097(98)00497-5. [DOI] [PubMed] [Google Scholar]
- 27.Grines CL, Cox DA, Stone GW, Garcia E, Mattos LA, Giambartolomei A, Brodie BR, Madonna O, Eijgelshoven M, Lansky AJ, O’Neill WW, Morice M-C The Stent Primary Angioplasty in Myocardial Infarction Study G. Coronary angioplasty with or without stent implantation for acute myocardial infarction. NEJM. 1999;341:1949–56. doi: 10.1056/NEJM199912233412601. [DOI] [PubMed] [Google Scholar]
- 28.Hamon M, Gomes S, Oppenheim C, Morello R, Sabatier R, Lognone T, Grollier G, Courtheoux P. Cerebral microembolism during cardiac catheterization and risk of acute brain injury: a prospective diffusion-weighted magnetic resonance imaging study. Stroke. 2006;37:2035–8. doi: 10.1161/01.STR.0000231641.55843.49. [DOI] [PubMed] [Google Scholar]
- 29.Busing KA, Schulte-Sasse C, Fluchter S, Suselbeck T, Haase KK, Neff W, Hirsch JG, Borggrefe M, Duber C. Cerebral infarction: incidence and risk factors after diagnostic and interventional cardiac catheterization—prospective evaluation at diffusion-weighted MR imaging. Radiology. 2005;235:177–83. doi: 10.1148/radiol.2351040117. [DOI] [PubMed] [Google Scholar]
- 30.Lund C, Nes RB, Ugelstad TP, Due-Tonnessen P, Andersen R, Hol PK, Brucher R, Russell D. Cerebral emboli during left heart catheterization may cause acute brain injury. Eur Heart J. 2005;26:1269–75. doi: 10.1093/eurheartj/ehi148. [DOI] [PubMed] [Google Scholar]
- 31.Talke P, Richardson CA, Scheinin M, Fisher DM. Postoperative pharmacokinetics and sympatholytic effects of dexmedetomidine. Anesth Analg. 1997;85:1136–42. doi: 10.1097/00000539-199711000-00033. [DOI] [PubMed] [Google Scholar]
- 32.Bustillo M, Lazar R, Finck A, Fitzsimmons B, Berman M, Pile-Spellman J, Heyer EJ. Dexmedetomidine impairs cognitive testing during endovascular embolization of cerebral arterio-venous malformations. J Neurosurg Anesthesiol. 2002;14:209–12. doi: 10.1097/01.ANA.0000017492.93942.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bekker A, Gold M, Ahmed R, Kim J, Rockman C, Jacobovitz G, Riles T, Fisch G. Dexmedetomidine does not increase the incidence of intracarotid shunting in patients undergoing awake carotid endarterectomy. Anesth Analg. 2006;103:955–8. doi: 10.1213/01.ane.0000237288.46912.39. [DOI] [PubMed] [Google Scholar]
- 34.Bekker AY, Basile J, Gold M, Riles T, Adelman M, Cuff G, Mathew JP, Goldberg JD. Dexmedetomidine for awake carotid endarterectomy: efficacy, hemodynamic profile, and side effects. J Neurosurg Anesthesiol. 2004;16:126–35. doi: 10.1097/00008506-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 35.McCutcheon CA, Orme RM, Scott DA, Davies MJ, McGlade DP. A comparison of dexmedetomidine versus conventional therapy for sedation and hemodynamic control during carotid endarterectomy performed under regional anesthesia. Anesth Analg. 2006;102:668–75. doi: 10.1213/01.ane.0000197777.62397.d5. [DOI] [PubMed] [Google Scholar]
- 36.Precedex™: redefining sedation, a Clinical Monograph Chicago, 2001
- 37.Connolly E, Winfree C, Rampersad A, Sharma R, Mack W, Mocco J, Solomon R, Todd G, Quest D, Stern Y, Heyer E. Serum S100B protein levels are correlated with subclinical neurocognitive declines after carotid endarterectomy. Neurosurgery. 2001;49:1076–83. doi: 10.1097/00006123-200111000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heyer EJ, DeLaPaz R, Halazun HJ, Rampersad A, Sciacca RR, Zurica J, Benvenisty A, Quest DO, Todd GJ, Lavine S, Solomon RA, Connolly ES., Jr Neuropsychological dysfunction in the absence of structural evidence for cerebral ischemia following uncomplicated carotid endarterectomy. Neurosurgery. 2006;58:474–80. doi: 10.1227/01.NEU.0000197123.09972.EA. discussion 80. [DOI] [PMC free article] [PubMed] [Google Scholar]