Figure 6.
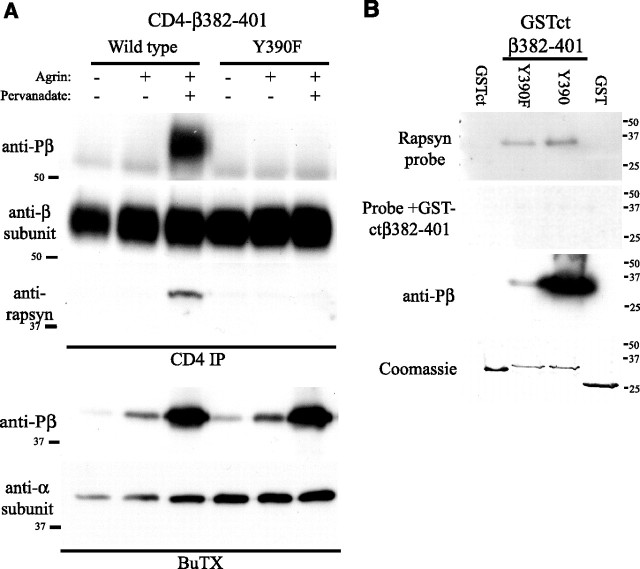
Rapsyn binds β loop 382–401 in a phosphorylation-dependent manner. A, CD4ct-β382–401 chimeras were immunoprecipitated from extracts of control or agrin plus pervanadate-treated myotubes (500 pm agrin, 0.2 mm pervanadate; 1 h) and immunoblotted with anti-rapsyn antibody (B5668). Rapsyn associated with wild-type CD4ct-β382–401 only after agrin plus pervanadate treatment that induced robust phosphorylation of Y390. No rapsyn associated with Y390F CD4ct-β382–401 that was expressed at similar levels but lacked phosphorylation (n = 4). Sequential isolation and immunoblotting of the AChR showed that agrin plus pervanadate induced significantly greater β subunit phosphorylation than agrin alone. B, GST fusion proteins immobilized on membranes were probed with 5 μg/ml GST-rapsyn and bound protein detected by immunoblotting with anti-rapsyn antibody (B5668). Rapsyn bound specifically to GSTct-β382–401 Y390 and Y390F but not to GST or GSTct controls, and binding was eliminated by incubating the GST-rapsyn probe together with 25 μg/ml soluble GSTct-β382–401. Coomassie staining shows that all fusion proteins were present at similar levels (n = 5).