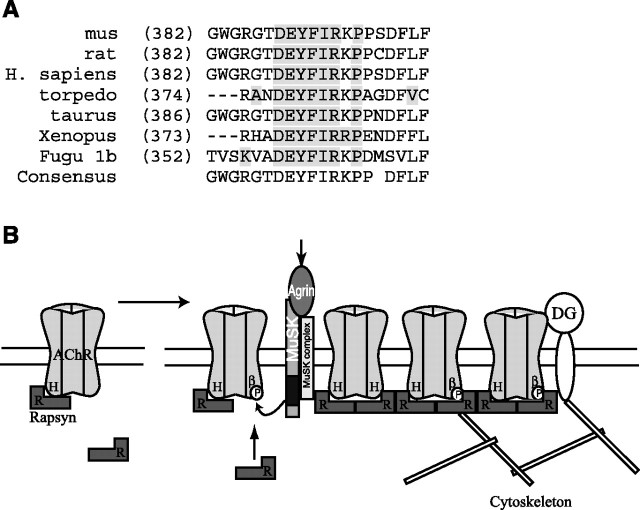
Figure 8.
Working model for how agrin-induced phosphorylation of AChR β subunit regulates rapsyn association and receptor localization. A, Alignment showing that the β subunit motif sufficient for clustering is highly conserved between species. B, Our data together with previous findings suggest a model in which AChR localization involves both constitutive and regulated interactions with rapsyn. In uninnervated muscle, rapsyn (R) is constitutively associated with the AChR, interacting with the helical domain (H) of one or more subunit loops. On innervation, agrin-induced phosphorylation of the AChR β subunit (βP) induces binding of additional rapsyn to the β 382–401 motif. This increase in stoichiometry may increase the efficiency of AChR clustering and also anchor and stabilize the receptor via interactions with dystroglycan (DG) or cytoskeletal proteins. Both forms of rapsyn binding contribute to localizing the AChR at high density in the postsynaptic membrane.