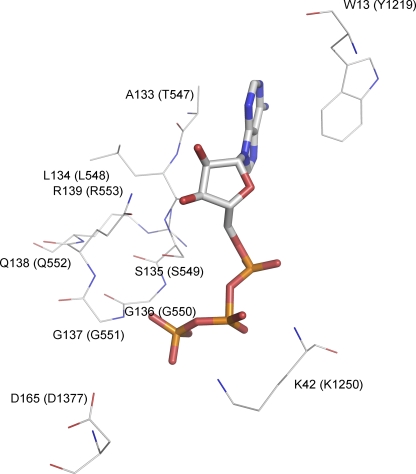
Figure 1.
Schematic presentation of the interactions of ATP with key residues in the ATP-binding pocket of an NBD dimer. The crystal structure of Escherichia coli Malk NBD dimer (Protein Data Bank code 1Q12) was used to demonstrate these interactions. The ATP molecule is represented by the stick model. Selective residues are shown and represented by thin lines, including residues of the signature sequence, the Walker A lysine, the D-loop aspartate, and the aromatic residue that strongly interacts with the adenine ring of ATP. These residues are colored by their atom types and labeled by their residue numbers as they appear in the amino acid sequence of E. coli Malk. The corresponding residues in CFTR are labeled in parentheses.