Figure 2. Covalent intermediate after the cleavage of nicotinamide.
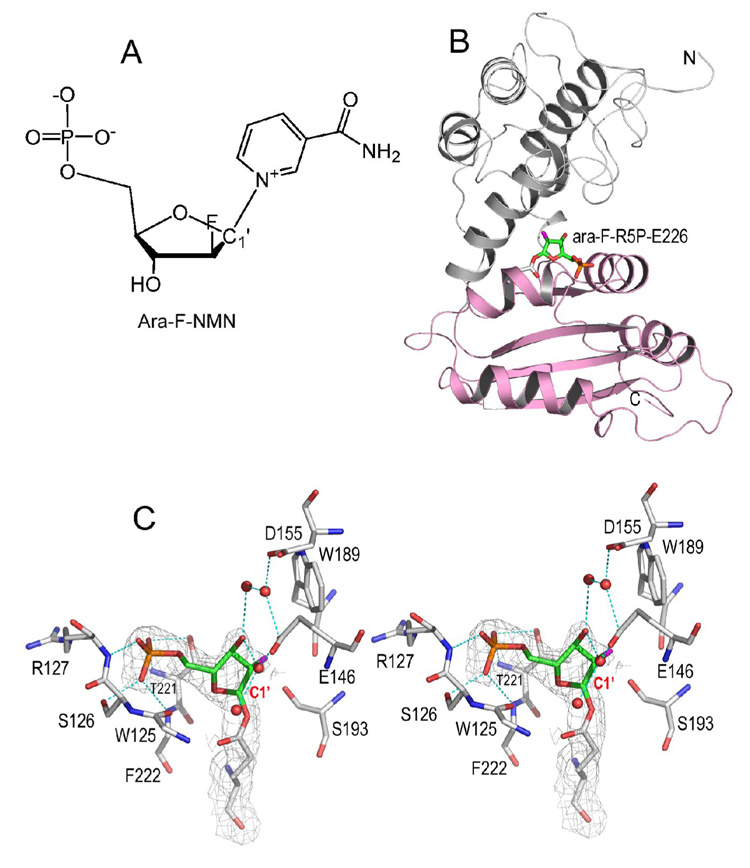
A) Chemical synthesized ara-F-NMN, a mechanism based analogue of substrate NMN. B) Overall structure of CD38 with its catalytic residue Glu226 covalently linked to ara-F-R5P. The two domains of CD38 are differently colored to show that the intermediate is right between the gulf of two domains. The covalent linkage is shown as sticks. C) A stereo presentation of active site structure showing the trapping of intermediate species ara-F-R5P. The 2Fo-Fc omit electron densities (with ara-F-R5P omitted during the calculation of the electron density map) for the trapped covalent intermediate is shown as gray isomesh at 1.0 σ. The active site residues are shown as sticks with their carbons atoms in gray. Ara-F-R5P, the remaining moiety after the release of leaving group nicotinamide from substrate, is also shown as sticks but with their carbon atoms in green. The covalent bond length between Glu226 and ara-F-R5P is 1.6 Å. Polar interactions between protein and ara-F-R5P are drawn as cyan dashed lines. Four water molecules observed in the active site are shown as red spheres.