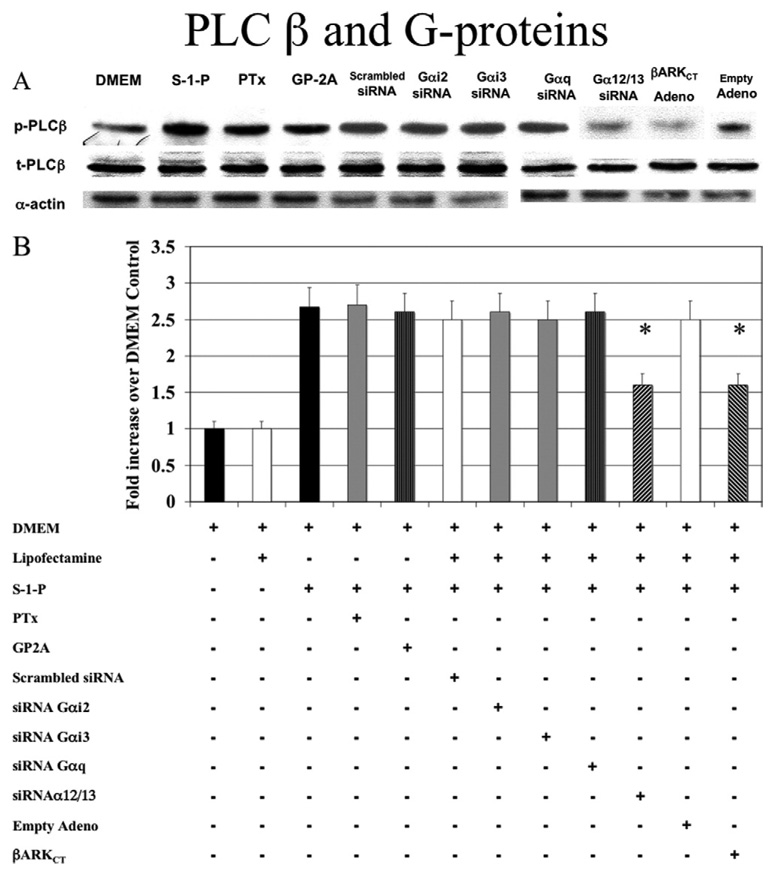
Fig. 2. G-proteins and phospholipase C-β (PLC-β).
A, Activation of PLC-β by sphingosine-1-phosphate (0.1µM) was Gαi-independent; that is, it was not blocked by pertussis toxin (PTx), a Gαi inhibitor, or Gαi2 and Gαi3 small interfering RNA (siRNA); and it was Gαq-independent (not blocked by GP-2A, a Gαq inhibitor, or Gαq siRNA). Small interfering RNA to Gα12/13 blocked PLC-β activation. Transfection with adenoviral COOH-terminus β-adrenergic receptor kinase (β-ARKCT) also blocked PLC-β activation. Representative Western blots are shown with α-actin as a loading control. B, The bar chart shows the quantification of the experiments representative in Fig 1, A and also shows additional controls. Scrambled siRNA and empty adenovirus did not significantly alter the response compared with control values. Values are the mean ± SEM of the ratio of the phosphorylation of PLC-β (p-PLC-β) relative to the total unphosphorylated PLC-β (t-PLC-β; n = 6). *P < .05 compared with control. DMEM, Dulbecco minimal essential medium; GP2A, glycoprotein 2A.