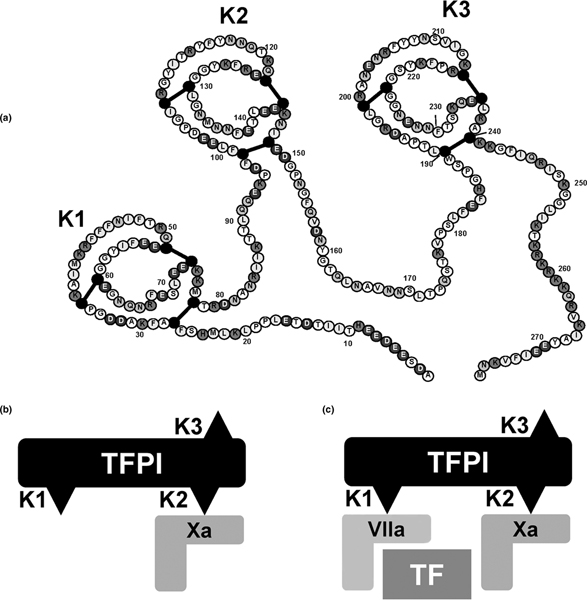
Figure 1.
The anticoagulant action of TFPI. (a) The structure of TFPIα. Adapted with permission from Monroe and Key [6]. The anticoagulant action of TFPI is a two-stage process. (b) The second Kunitz (K2) domain binds first to a molecule of Xa and deactivates it. (c) The first Kunitz domain (K1) rapidly binds to an adjacent TF-VIIa complex, preventing further activation of X. The formation of this quaternary compound is necessary for the inhibitory action of TFPI on the TF-VIIa complex. TFPI can bind TF-VIIa, but this interaction is weak. The TFPI-Xa interaction is strong, whereas the interaction between TFPI-Xa and TF-VIIa is very strong and results in an essentially irreversible complex: TFPI-Xa-TF-VIIa. TF, tissue factor; TFPI, tissue factor pathway inhibitor; VIIa, activated factor VII; X, factor X; Xa, activated factor X.