Abstract
Background/Objectives:
Knowledge of spinal cord injury (SCI) bone changes has been derived primarily through cross-sectional studies, many of which are controvertible. Longitudinal studies are sparse, and long-term longitudinal chronic studies are unavailable. The objective of this study was to provide a clearer perception of chronic longitudinal bone variations in people with complete SCI.
Methods:
Bone status of 31 individuals with chronic, complete SCI was assessed twice using dual-energy x-ray absorptiometry at an average interval of 5.06 ± 0.9 years. Because the sample of women was small (4), the primary analyses of change and comparisons of those with paraplegia vs tetraplegia were confined to the male participants.
Results:
Spine Z-scores showed a significant increase (P < 0.0001). The average Z-scores, initial and follow-up, were within the normal range. Hip Z-scores also showed a significant increase (P < 0.0001), and hip bone mineral density (BMD) increased in 48% of the participants. Knee BMD and lower extremity total bone mineral showed significant decreases (P < 0.003 and P < 0.02, respectively), but increases were seen in 33% and 26% at the respective sites. Individuals with tetraplegia had significantly lower values across all regions (P < 0.0001), and changes were significantly different compared with paraplegia (P < 0.0001). Bone values and changes in men vs women, despite the small sample of women, showed highly significant differences (P < 0.003–0.002).
Conclusion:
Chronic effects of complete SCI do not exclusively result in continued loss of BMD or a static state of lowered BMD; gain in BMD may occur. The nature and magnitude of the effects of complete SCI on BMD vary by site, with sex and level of injury, which has implications for treatment and its assessment.
Keywords: Spinal cord injuries, chronic; Tetraplegia; Paraplegia; Osteoporosis; Bone loss; Fractures
INTRODUCTION
Bone mineral density (BMD) changes related to spinal cord injury (SCI) have been divided into 3 general phases (1). The acute or “response to injury” phase occurs during the first 4 months or so after injury. BMD at the knee decreases at a rate of 1% per week, whereas BMD at the pelvis and os calcis and total bone mineral (TBM) in the entire lower extremity (LE) decreases at a rate of 2% per week (2–4). This rapid BMD loss corresponds to elevation of bone resorption markers in the serum and urine. The dramatic osteoclastic response is detected by hypercalciuria, a nonspecific bone resorption marker (hydroxyproline), and specific markers: deoxypyridinoline, pyridinoline, and N-telopeptide. All of these indicators become elevated soon after injury and peak at approximately 24 weeks after injury (5).
The subacute or “adaptation and adjustments” phase ensues thereafter and persists for another year, more or less (3,4). Many of the bone resorption markers have or are returning to normalcy, whereas endocrine responses, such as parathyroid (PTH), may have become elevated but are also returning to baseline. Markers for osteoblastic activity have for the most part failed to show an increase or decrease in activity during these 2 periods (6–13). The BMD loss at the knee decreases to 1% per month, whereas rapid loss persists at the os calcis and entire LE (3,4,14).
Few acute or chronic longitudinal studies of bone loss in this population are available, perhaps principally because of the small size of this population (15–20). Interfacility transfers after acute injury and a paucity of centers for chronic care in the United States, excluding the Veteran Health Administration, also render data collection difficult. There have been reports on the longitudinal decrease in BMD in men with complete SCI in acute and subacute states (2–4). The purpose of this report is to document and provide clearer perception of chronic longitudinal BMD variations in men (and a small sample of women) with complete SCI.
METHODS
Participants
Thirty-one individuals (27 men and 4 women) with chronic, complete SCI participated in the study, including 16 with paraplegia (13 men and 3 women) and 15 with tetraplegia (14 men and 1 woman). Age at initial examination was 39.7 ± 10.6 years. Duration of injury (DOI) at initial examination was 14.6 ± 8.7 years, with re-examination at 5.06 ± 0.9 years. The initial mean age and DOI of men was 38.5 ± 9.2 and 13.3 ± 8.1 years, respectively, with re-examination at 5.07 ± 0.9 years. The initial mean age and DOI of women was 48.0 ± 16.4 and 19.8 ± 12.1 years, respectively, with re-examination at 5.0 ± 0.8 years.
Procedures
Dual-energy x-ray absorptiometry (DXA; QDR-2000; Hologic, Waltham, MA) was used to measure BMD at the spine, hip, and knee and TBM in the LE. This procedure also provides age-, sex-, and race-normalized Z-scores for the spine and hip. BMD at the knee was measured with modification of the FM-1 Forearm Application Protocol Software (Hologic) (21). The distal femur and proximal tibia, as well as their net mean, which provides a BMD value for the knee, were assessed. All participants had full initial and follow-up BMD data for the spine, hip, distal femur, and proximal tibia, as well as TBM data for the LE. Measures for the hip, distal femur, and proximal tibia, as well as LE data, were taken from the dominant hand side based on participant self-report.
To support the long-term reproducibility and measurement reliability, a standard phantom was scanned before each procedure, and the machine was recalibrated accordingly to adjust for potential machine and radiation source changes. For follow-up scanning, anatomical landmarks were used for positioning of the individual being scanned by the technician performing the procedure. These protocols were used for clinical and research procedures. To further support measurement reliability, all scans performed for this study were done by the same highly experienced technician.
Regional results produced a visual radiographic scan and data. These scans and data were reviewed by a radiologist (C.A.S.). Individuals with degenerative changes or ossifications were excluded from the study, as were those with previous fractures at a measurement site, which may have occurred before, concurrent with, or after SCI. Individuals with instrumentation/fixation devices that interfered with measurement were also excluded from the study.
Statistical Methods
Repeated-measures analyses of covariance controlling for age and DOI were used to assess BMD at all sites, because age and DOI influence bone loss (22) and because there were wide ranges in age and DOI represented in the sample. The primary analyses of change and comparison of paraplegia vs tetraplegia were confined to the male participants because of the small sample of women and because women with SCI have shown different BMD loss patterns than men (23). The period between initial and follow-up testing was relatively consistent across participants, but it was also controlled in the analyses of covariance. Repeated-measures analyses of covariance controlling only for DOI and follow-up time were used for Z-score data (hip and spine), which are population SDs normalized for race, sex, and age. Only 1 woman had tetraplegia; therefore, the only sex comparisons were made between men and women with paraplegia.
RESULTS
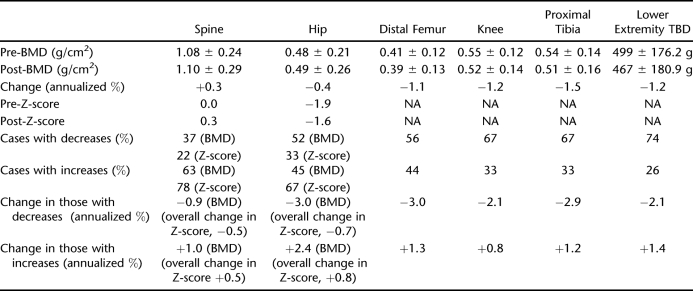
Table 1 provides a summary of the changes observed across the regions studied.
Table 1.
Longitudinal Changes in Bone Across Regions
Spine
The mean spine BMDs increased from initial and follow-up measurement points. This increase approached but did not achieve significance (P = 0.095). Spine BMD increased vs decreased in nearly two thirds of the participants. The relative/absolute mean annualized percentage of change was greater for those whose spine BMD increased vs decreased. The mean spine Z-scores also increased from initial and follow-up measurement points and increased in more than three quarters of the participants.
Comparison of individuals with spine BMD or Z-score increases vs decreases did not show differences in age, injury duration, or amount of time from initial to follow-up examination. No statistical differences in spine BMD or Z-score were noted between paraplegia and tetraplegia.
Hip
Mean hip BMDs increased from initial and follow-up measurement points. The hip BMD increase approached but did not achieve significance (P = 0.066). The hip BMD decreased vs increased in a slightly greater percentage of the participants. The relative mean annualized percent change was greater for those with decreases vs increases. The hip BMD decreases occurred in significantly younger individuals compared with those with increases: 32.7 ± 7.4 vs 44.8 ± 6.7 years at initial examination (P = 0.0002) and 37.7 ± 7.1 vs 49.9 ± 7.0 years at follow-up examinations (P = 0.0001). The hip BMD decreases occurred in individuals who had been injured a significantly shorter duration than those whose hip BMD increased: 10.2 ± 7.8 vs 16.7 ± 7.1 years at initial examination (P = 0.0341) and 15.2 ± 7.2 vs 21.8 ± 7.3 years at follow-up examination (P = 0.0259).
There was a significant difference between initial and follow-up mean hip Z-scores (P < 0.0001), reflecting an improvement at follow-up (ie, movement closer to the norm standardized for age, race, and sex). The hip Z-score increased in two thirds of the participants. The relative mean change in Z-scores was greater for those who showed positive changes in Z-score. Hip Z-score decreases were in significantly younger individuals compared with those whose hip Z-score (increased: 32.1 ± 8.2 vs 41.7 ± 8.1 years at initial examination [P = 0.008]; 37.0 ± 7.9 vs 46.9 ± 8.2 years at follow-up examinations [P = 0.0063]). However, DOI was not significantly different for decreased vs increased hip Z-scores. Neither time from initial to follow-up examination nor having paraplegia vs tetraplegia was related to decreases vs increases in hip BMD or Z-scores.
Knee
Initial mean knee BMDs were significantly lower than those at follow-up (P < 0.003). The distal femur and proximal BMDs were also lower at follow-up (P < 0.056 and P < 0.015, respectively). Mean annualized changes were greatest at the proximal tibia and least at the distal femur. Net average knee BMD decreased in two thirds of the participants. Relative mean annualized percent change in knee BMD was greater in those who showed a decrease vs increase. Average BMD of the distal femur decreased in slightly more than one half of the participants. Average BMD of the proximal tibia decreased in two thirds of the participants (same as the net average of the knee BMD). Relative mean annualized percent changes in distal femur and proximal tibia BMD was greater for those who showed decreases.
Lower Extremity Total Bone Mineral
Mean LE TBM was significantly lower at follow-up (P = 0.02). TBM decreased in nearly three quarters of the participants. The relative mean annualized percentage of change was greater in individuals with decreases in LE TBM. Age, injury duration, time from initial to follow-up examination, or having paraplegia vs tetraplegia were unrelated to decrease vs increases in knee, distal or proximal tibia BMD, or LE TBM.
Figure 1 shows the relative annualized percents of change across the regions studied. Although the annualized percent of change in the spine was a positive 0.3% and the annualized percent of change in the hip was a negative 0.4%, the overall change in spine BMD only approached significance, and the overall change in hip BMD was positive but insignificant as described above. The annualized percentages of change about the knee were consistent with the overall significant changes in BMD, with greater loss of BMD proximal to distal, ranging from a mean of −1.1% to a mean of −1.5%. The annualized percentage of change in the LE, negative 1.2%, was also consistent with the overall significant decrease in TBM of the LE.
Figure 1. Annualized percent of change.
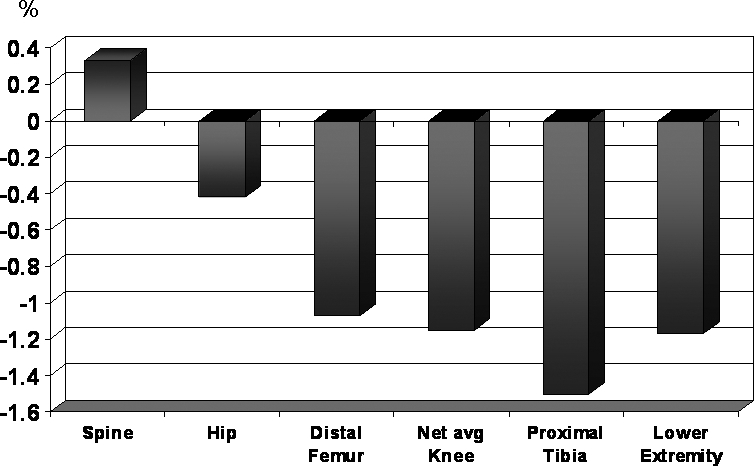
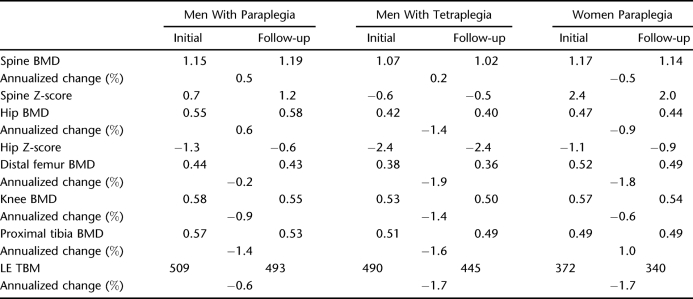
Table 2 provides a summary of the changes observed across the regions and differences associated with impairment and sex as reported below.
Table 2.
Longitudinal Bone Changes by Impairment and Sex
Impairment
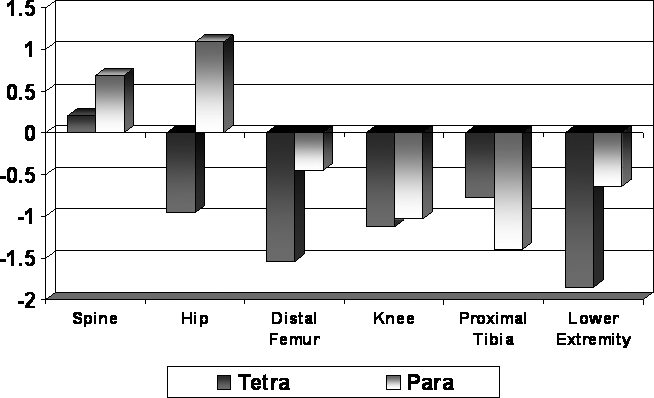
Individuals with tetraplegia had significantly lower BMD and LE TBM values across all regions (P < 0.0001; Figure 2). Individuals with tetraplegia had significantly lower Z-scores than those with paraplegia for both spine and hip, with similar changes in BMD values. Changes from initial to follow-up examination in each region were significantly different for tetraplegia vs paraplegia (P < 0.0001; Figure 3).
Figure 2. BMD changes at primary regions.
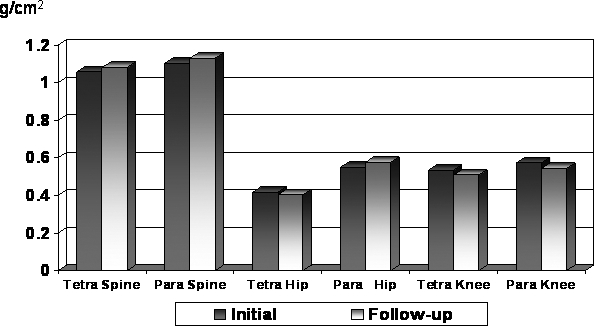
Figure 3. Annualized percent change: regional comparisons by impairment.
Sex
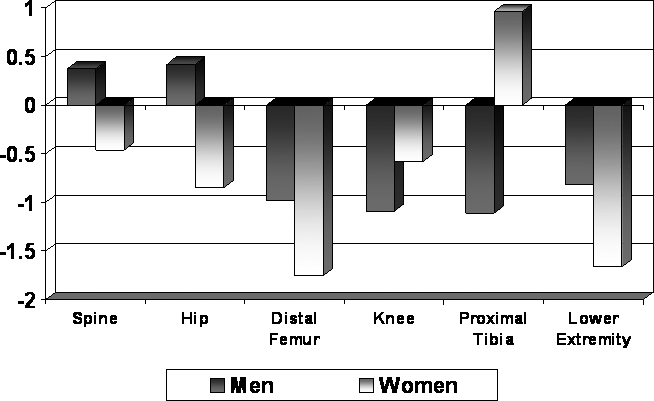
Comparisons between men and women with complete paraplegia, despite the small sample of women, showed highly significant sex differences: spine, hip, and knee (P = 0.002); spine and hip Z-scores; and LE TBM (P = 0.003). Nevertheless, the very small sample of women makes generalization tenuous. Women had lower BMD values across all regions at initial and follow-up examinations (Figure 4). This was also true for the LE TBM and hip Z-scores (data not shown). This was, however, not true for the spine Z-score. The mean spine Z-scores for women decreased from initial to follow-up examination but increased for men. The changes in BMD and Z-scores for women at each region from initial to follow-up examination were significantly different from the changes observed in men (P < 0.0001); often the changes were in the opposite direction, such as the spine Z-scores shown in Figure 5.
Figure 4. BMD changes at primary regions.
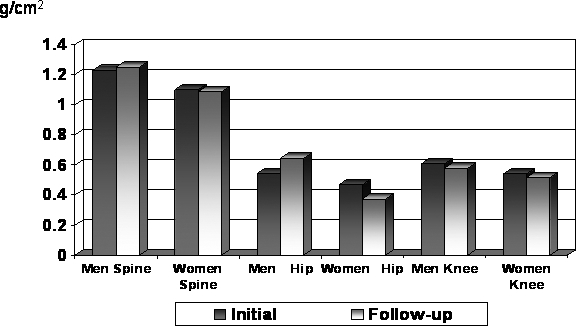
Figure 5. Annualized percent change: regional comparisons.
DISCUSSION
The chronic or “impairment” phase begins around 1.5 to 2 years after injury. Most resorptive and endocrine markers have returned to baseline. Cross-sectional studies have shown annual BMD loss at the hip is −0.5% and is −1.0% at the knee (1,2,4). To date, most studies detailing the BMD changes have been cross-sectional. In addition, many have combined acute and chronic losses, used mixed samples of men and women, and neurologic impairments (paraplegia and tetraplegia, complete and incomplete lesions) (24–28). This study documents longitudinal BMD changes in the spine and LE.
A BMD loss initially occurs at the lumbar spine of approximately 10%, but this is followed by a net gain during the ensuing 10 or more years, the latter having been well documented by cross-sectional studies (4,15,24–29). This study confirmed that overall BMD is gained chronically in the SCI lumbar spine. Our study interval showed a higher value BMD and Z-score at the lumbar spine in both women (1.14 g/cm2, 2.0 Z-score) and men (1.19 g/cm2, 1.2 Z-score) with complete paraplegia, which is higher than their average able-bodied counterparts. The women, however, actually began to slowly lose BMD again annually (−0.5%), although their values remained 2 SD above the norm for race and age. The annual increase in spine BMD was greater in men with paraplegia (+0.5%) than in men with tetraplegia (+0.2%), although both had positive gains, with the men with paraplegia remaining 1 SD above their able-bodied peers, and the men with tetraplegia with values within the norm and improving.
These lumbar spine data support the clinical fact that spine compression fractures in this population are either underreported or are uncommon. Without additional underlying, non–SCI-related pathology, the lumbar spine should never reach fracture threshold and fracture despite compressive forces of the active, self-propelled individual with paraplegia. This natural history of BMD accretion also renders the lumbar spine a poor site for treatment assessment and modality in this population, although it is the primary study site for treatment assessment and modality in the segment of the general population most affected by pathologic bone loss, postmenopausal women. The natural history of BMD gain in the chronic phase may make treatment BMD endpoints spuriously positive.
Women with complete SCI, as well men with tetraplegia, may go through a cycle of loss, gain, and loss, depending on their DOI. Men with paraplegia may continue to have a net gain. Overall, 63% (17 of 27) of men gained lumbar spine BMD.
The acute BMD loss at the hip is 0.5% weekly in men with complete injuries and 0.5% monthly during the subacute phase (1). It can be extrapolated that BMD loss will total 15% to 20% by 1.5 to 2 years after injury (4). Chronically, however, the hip develops some characteristics germane to the lumbar spine (BMD gain), but also of the distal LE (BMD loss). The chronic mean annualized change was −0.4%. Women with complete paraplegia lost 0.9% per year and men with tetraplegia lost 1.4% per year. Men with complete paraplegia gained 0.6% annually, perhaps by a similar mechanism for increasing BMD in the lumbar spine. Men with tetraplegia and women with paraplegia continue to lose at the hip similar to the lumbar spine in women with longstanding paraplegia.
The hip Z-scores were relatively unchanged in men with tetraplegia but improved in both men and women with paraplegia (Table 2). The hip lost BMD in some whereas gaining in others, and the hip Z-score improved in two thirds of the sample. This has important clinical implications regarding spuriously positive treatment BMD endpoints, as was noted in the lumbar spine. The tetraplegic hip is nearly osteoporotic and at fracture threshold, whereas the paraplegic hip is not. However, individuals with tetraplegia do not experience fracture as frequently as individuals with paraplegia, perhaps because of their lack of exposure to risk (ie, mobility and transfers) despite low hip BMD. The person with paraplegia, however, may not fracture the hip as often as older literature suggests (30–34). The incidence of hip fracture reported in the past has been generated by hospital-based studies. Because the paraplegic hip is not at fracture threshold, it should not fracture as often as other LE locations. A recent outpatient-based fracture survey supports this assertion (2). We believe the vagaries of the data (loss vs gain) caused by sex, DOI, and neurologic impairment also render the hip a potentially poor study site to monitor the effects of treatment based on hip BMD. This is disappointing because hip DXA norms and procedures are readily available, and the hip has been suggested as the best site for predicting hip fracture after treatment in the general population (35).
The knee has a rapid decrease of BMD during the acute and subacute phases, with men losing up to 30% of their BMD in 1.5 to 2 years, whereas women lose more (1–4,23). Chronically, the annual BMD loss was 1.1% at the distal femur, 1.2% at the knee, and 1.5% at the proximal tibia. Men lose more BMD at the knee than women annually (1.2% vs 0.6%), but all women lost BMD, whereas one third of the men's knees gained BMD. The annual tetraplegic loss was 1.4%, whereas the paraplegic loss was 0.9%. This is consistent with previous reports (4,23,24).
Women with paraplegia and men with tetraplegia lost BMD at the hip but some men with paraplegia gained BMD. However, the decrease in hip BMD among those with paraplegia who showed decline was less than the decline observed in those with tetraplegia.
LE TBM, as well as BMD of the os calcis and pelvis, decreases more rapidly than all other sites in SCI (1,3,4,14). BMD is lost progressively in a proximal to distal fashion (excluding the pelvis). Bone mineral loss from the entire LE is reflected differently. TBM loss herein is 2% weekly, both acutely and subacutely (1). Chronically, however, the annualized LE TBM loss decreased to 1.2% in men. Men and women with paraplegia lost 0.6% and 1.7%, respectively, whereas men with tetraplegia lost 1.7%. Even here, however, it seems that the LE can gain bone mineral as happened in 26% (7 of 27) of the men. This may reflect hip and knee increments as well as increments below the knee or the gains at the hip and knee are larger than the losses below the knee. Chronic longitudinal studies focusing on the os calcis are not available to assist in further definition of this phenomenon.
It has been previously reported that completeness of injury was the most important risk factor for BMD loss and that individuals with complete injury had BMD loss greater than individuals with incomplete injury (2,21,22). The men with tetraplegia have lower BMD and Z-scores in all areas compared with their paraplegic counterparts. Furthermore, BMD loss predictably occurred in men with tetraplegia, whereas some men with paraplegia showed BMD gains.
Few studies have included sex as a factor for BMD loss in SCI (3,23). Despite the small number of women with complete paraplegia in this study, which makes generalization tenuous, comparisons with their male counterparts are quite robust and consistent with previous findings. Sex differences were noted at the spine and hip BMD, as well as the Z-scores, knee BMD, and LE TBM. The regions studied generally had lower BMDs and larger decreases in BMD during the study period.
The ultimate endpoint for treatment of pathologic bone loss is fracture reduction. Breaking strength of bone is linearly related to its bone mineral content, and its measurement at fracture sites determines fracture risk (36). The level of BMD in a specific site below which most fractures occur (minus major trauma) has been termed “fracture threshold.” The prevalence and relative risk of fracture increases dramatically with decreasing BMD, whereas it is unlikely above the threshold density. A majority of postmenopausal pathological fractures occur at the spine and hip (femoral neck and intertrochanteric region of the femur). Their assigned fracture threshold values were established at 0.97, 0.95, and 0.92 g/cm2, respectively (Z-scores of −2.3, −2.4, and −2.2, respectively) (36). A further refinement of this concept established BMD fracture thresholds, a point wherein fractures begin occurring and BMD “fracture breakpoints” at values when the majority of fractures occur (35).
Fracture thresholds, T- or Z-scores, above the knee have been established in the able-bodied population; but unfortunately, not at the knee or below. However, the SCI population frequently sustains fractures about the knee (30–34). Therefore, we, as well as others, have attempted to define it (37–41). We believe the fracture threshold at the knee is 0.87 g/cm2, with a fracture breakpoint of 49 g/cm2 (approximately 50% decrease in BMD) (38–41). We considered the os calcis as a site to study treatment, but this site rarely sustains fractures, negating an important endpoint (2,3).
This study, in conjunction with our previous studies and others, may assist in our understanding of the natural history of SCI osteoporosis, indications for treatment, and treatment interpretation. Acutely, the knee of the man with complete SCI loses 1% BMD per week (16%) over 4 months; subacutely, it loses 1% BMD per month (12%) over 12 months. The knee sustains a 28% decrease in BMD (more or less) by 1.5 to 2 years. If the knee BMD continues to decrease at the rate of 1% annually, 20 years after injury, many men with complete paraplegia will be in the vicinity of fracture breakpoint (16% + 12% + 18% = 46%). This places them below fracture threshold and slightly above fracture breakpoint. Certainly, if this person fractures his extremity, he is at risk for another fracture (38–41).
This study indicates that most women with complete paraplegia and men with complete tetraplegia will reach fracture threshold at the knee within a few years after injury and progress to fracture breakpoint. Men with complete paraplegia may reach fracture threshold without progressing to fracture breakpoint. However, we do not know which group of men with SCI will gain BMD and which will lose BMD. Eventually, treatment of SCI osteoporosis should begin before reaching fracture threshold, especially in women with complete paraplegia and men with complete tetraplegia, and probably in women with complete tetraplegia as well. A positive change in BMD at the knee may indicate treatment is effective in women with complete injuries and in men with complete tetraplegia but may be part of normal waxing and waning of BMD in men with complete paraplegia.
We understand that much of our work may be spurious because of small samples, machine precision error, modified software, and peripheral testing. The World Health Organization (WHO) based their diagnosis of osteoporosis, osteopenia, and fracture risk assessment on central DXA of the hip (42). Peripheral BMD technologies, excluding wrist peripheral BMD devices, were not used in the database for establishing WHO criteria. Many T-scores derived from peripheral BMD devices are derived from their own manufacturer-specific and inconsistent young (“normal”) reference population databases (43,44). This may leave our distal femur, proximal tibia, and knee BMD data open to question.
CONCLUSION
The chronic effects of complete SCI do not exclusively result in continued loss of BMD or a static state of lowered BMD; gain in BMD may occur. The nature and magnitude of the effects of complete SCI on BMD vary by site with sex and level of injury and are associated with duration of the injury. The spine and hip, anatomical sites commonly used to assess of the effectiveness of treatment modalities in the non-SCI population, are not appropriate anatomical study sites for the assessment of treatment modalities for individuals with SCI. Assessment of treatment modality effectiveness for those with complete SCI should be based on studies of the effects on the knee. Clinical assessment of fracture risk and treatment decisions should take into account the BMD proximity to fracture threshold.
REFERENCES
- Garland DE, Adkins RH, Stewart CA. The natural history of bone loss in the lower extremity of complete spinal cord injured males. Top Spinal Cord Inj Rehabil. 2005;11:48–60. [Google Scholar]
- Garland DE, Adkins RH. Bone loss at the knee in spinal cord injury. Top Spinal Cord Inj Rehabil. 2001;6:37–46. [Google Scholar]
- Garland DE, Adkins RH, Scott M, Singh H, Massih M, Stewart CA. Bone loss at the os calcis compared with bone loss at the knee in individuals with spinal cord injury. J Spinal Cord Med. 2004;27:207–211. doi: 10.1080/10790268.2004.11753749. [DOI] [PubMed] [Google Scholar]
- Garland DE, Stewart CA, Adkins RH, Hu SS, Rosen C, Liotta FJ, Weinstein DA. Osteoporosis after spinal cord injury. J Orthop Res. 1992;10:371–378. doi: 10.1002/jor.1100100309. [DOI] [PubMed] [Google Scholar]
- Roberts D, Lee W, Cunco RC, et al. Longitudinal study of bone turnover after acute spinal cord injury. J Clin Endocrinol Metab. 1998;83:415–422. doi: 10.1210/jcem.83.2.4581. [DOI] [PubMed] [Google Scholar]
- Chen B, Stein A. Osteoporosis in acute spinal cord injury. Top Spinal Cord Inj Rehabil. 2003;9:26–35. [Google Scholar]
- Finsen V, Indredavik B, Fougner KJ. Bone mineral and hormone status in paraplegics. Paraplegia. 1992;30:343–347. doi: 10.1038/sc.1992.80. [DOI] [PubMed] [Google Scholar]
- Maemorin L, Couret I, Micallef JP, et al. Use of biochemical markers with dual energy x-ray absorptiometry for early determination of bone loss in persons with spinal cord injury. Metabolism. 2002;51:958–963. doi: 10.1053/meta.2002.34013. [DOI] [PubMed] [Google Scholar]
- Maynard FM. Immobilization hypercalcemia following spinal cord injury. Arch Phys Med Rehabil. 1986;67:41–44. [PubMed] [Google Scholar]
- Pietschmann P, Pils P, Woloszczuk W, Maerk R, Lessen D, Sitpicic U. Increased serum osteocalcin levels in patients with paraplegia. Paraplegia. 1992;30:204–209. doi: 10.1038/sc.1992.56. [DOI] [PubMed] [Google Scholar]
- Uebelhart D, Demiaux-Domenech B, Roth M, Chantraine A. Bone metabolism in spinal cord injured individuals and in others who have prolonged immobilization. A review. Paraplegia. 1995;33:669–673. doi: 10.1038/sc.1995.140. [DOI] [PubMed] [Google Scholar]
- Uebelhart D, Hartmann D, Vuagnat H, Castainer M, Hachen HJ, Chantraine A. Early modifications of biochemical markers of bone metabolism in spinal cord injury patients. A preliminary study. Scand J Rehabil Med. 1994;26:197–202. [PubMed] [Google Scholar]
- Zender Y, Luthi M, Michael D, et al. Long term changes in bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: a cross-sectional observational study in 100 paraplegic men. Osteoporos Int. 2004;15:180–189. doi: 10.1007/s00198-003-1529-6. [DOI] [PubMed] [Google Scholar]
- Chow YW, Inman C, Sharp CA, Haddaway MJ, El Masry W, Davie MWJ. Ultrasound bone densitometry and dual energy x-ray absorptiometry in patients with spinal cord injury: a cross-sectional study. Spinal Cord. 1996;34:736–741. doi: 10.1038/sc.1996.134. [DOI] [PubMed] [Google Scholar]
- Biering-Sorensen F, Bohr H, Schaadt O. Longitudinal study of bone mineral content in the lumbar spine, the forearm and the lower extremities after spinal cord injury. Eur J Clin Invest. 1990;20:330–335. doi: 10.1111/j.1365-2362.1990.tb01865.x. [DOI] [PubMed] [Google Scholar]
- de Bruin ED, Dietz V, Dambacher MA, Stussi E. Longitudinal changes in bone in men with spinal cord injury. Clin Rehabil. 2000;14:145–152. doi: 10.1191/026921500670532165. [DOI] [PubMed] [Google Scholar]
- Frey-Rindova P, de Bruin Ed, Stussi E, Dambacher MA, Dietz V. Bone mineral density in upper and lower extremities during 12 months after spinal cord injury measured by peripheral quantitative computed tomography. Spinal Cord. 2000;38:26–32. doi: 10.1038/sj.sc.3100905. [DOI] [PubMed] [Google Scholar]
- de Bruin ED, Vanwanseele B, Dambacher MA, Dietz V, Stussi E. Long-term changes in the tibia and radius bone mineral density following spinal cord injury. Spinal Cord. 2005;43:96–101. doi: 10.1038/sj.sc.3101685. [DOI] [PubMed] [Google Scholar]
- Warden SU, Bennell KL, Matthews B, Brown DJ, McMeehen JM, Wark JD. Quantitative ultrasound assessment of acute bone loss following spinal cord injury: a longitudinal pilot study. Osteoporosis. 2000;13:586–592. doi: 10.1007/s001980200077. [DOI] [PubMed] [Google Scholar]
- Wilmet E, Ismail AA, Heilporn A, Welraeds D, Bergmann P. Longitudinal study of the bone mineral content and soft tissue composition after spinal cord section. Paraplegia. 1995;33:674–677. doi: 10.1038/sc.1995.141. [DOI] [PubMed] [Google Scholar]
- Garland DE, Foulkes GD, Adkins RH, Stewart CA, Yakura JS. Regional osteoporosis following incomplete spinal cord injury. Contemp Orthop. 1994;28:134–139. [Google Scholar]
- Garland DE, Adkins RH, Rah A, Stewart CA. Bone loss with aging and the impact of SCI. Top Spinal Cord Inj Rehabil. 2001;6:47–60. [Google Scholar]
- Garland DE, Adkins RH, Stewart CA, Ashford R, Vigil D. Regional osteoporosis in women who have a complete spinal cord injury. J Bone Joint Surg Am. 2001;83:1195–1200. doi: 10.2106/00004623-200108000-00009. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM, Wang J, Pierson RN, Jr, Schwartz E. Continuous loss of bone during chronic immobilization: a monozygotic twin study. Osteoporos Int. 1999;10:123–127. doi: 10.1007/s001980050206. [DOI] [PubMed] [Google Scholar]
- Leslie WD, Nance PW. Dissociated hip and spine demineralization: a specific finding in spinal cord injury. Arch Phys Med Rehabil. 1993;74:960–964. [PubMed] [Google Scholar]
- Sabo D, Blaich S, Wenz W, Hohmann M, Loew M, Gerner HJ. Osteoporosis in patients with paralysis after spinal cord injury. A cross sectional study in 46 male patients with dual-energy absorptiometry. Arch Orthop Trauma Surg. 2001;121:75–78. doi: 10.1007/s004020000162. [DOI] [PubMed] [Google Scholar]
- Szollar SM, Martin EM, Parthemore JG, Sartoris DJ, Deftos LJ. Demineralization in tetraplegic and paraplegic man over time. Spinal Cord. 1997;35:223–228. doi: 10.1038/sj.sc.3100401. [DOI] [PubMed] [Google Scholar]
- Szollar SM, Martin EM Parthemore JG, Sartoris DJ, Deftos LJ. Densitometric patterns of spinal cord injury associated bone loss. Spinal Cord. 1997;35:374–382. doi: 10.1038/sj.sc.3100394. [DOI] [PubMed] [Google Scholar]
- Dauty M, Perroulin Verbe B, Maugars Y, Dubois C, Mathe JF. Supralesional and sublesional bone mineral density in spinal cord injured patients. Bone. 2000;27:305–309. doi: 10.1016/s8756-3282(00)00326-4. [DOI] [PubMed] [Google Scholar]
- Comarr AE, Hutchinson RH, Bors EB. Extremity fractures of patients with spinal cord injuries. Am J Surg. 1962;103:732–739. doi: 10.1016/0002-9610(62)90256-8. [DOI] [PubMed] [Google Scholar]
- Eichenholtz SN. Management of long-bone fracture in paraplegic patients. J Bone Joint Surg Am. 1963;45:299–310. [Google Scholar]
- Freehafer AA, Hazel CM Becker CL. Lower extremity fractures in patients with spinal cord injury. Paraplegia. 1981;19:367–372. doi: 10.1038/sc.1981.69. [DOI] [PubMed] [Google Scholar]
- Freehafer AA, Mast WA. Lower extremity fractures in patients with spinal cord injury. J Bone Joint Surg Am. 1965;47:683–694. [PubMed] [Google Scholar]
- McMaster WC, Stauffer ES. The management of long bone fracture in the spinal cord injured patient. Clin Orthop Rel Res. 1975;112:44–52. [PubMed] [Google Scholar]
- Mazess RB. Bone densitometry of the axial skeleton. Orthop Clin North Am. 1990;21:51–63. [PubMed] [Google Scholar]
- Riggs BL, Wahner HW, Dunn WL, Mazess RB, Offord KP, Melton LJ., III Differential changes in bone mineral density of the appendicular and axial skeleton with aging: relationship to spinal stenosis. J Clin Invest. 1981;67:328–335. doi: 10.1172/JCI110039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eser P, Frotzler A, Zehnder Y, Denoth J. Fracture threshold in the femur and tibia of people witih spinal cord injury as determined by peripheral quantitative computed tomography. Arch Phys Med Rehabil. 2005;86:498–504. doi: 10.1016/j.apmr.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Garland DE, Adkins RH, Kushwaha V, Stewart C. Risk factors for osteoporosis at the knee in the spinal cord injury population. J Spinal Cord Med. 2004;27:202–206. doi: 10.1080/10790268.2004.11753748. [DOI] [PubMed] [Google Scholar]
- Garland DE, Adkins RH, Stewart CA. Fracture threshold and risk for osteoporosis and pathological fracture individuals with spinal cord injury. Top Spinal Cord Inj Rehabil. 2005;11:61–69. [Google Scholar]
- Garland DE, Maric Z, Adkins RH, Stewart CA. Bone density about the knee in spinal cord injured patients with pathologic fractures. Contemp Orthop. 1993;26:375–379. [Google Scholar]
- Lazo MC, Shirazi P, Sam M, Giobbie-Hurder A, Blacconier MJ, Muppidi M. Osteoporosis and risk of fracture in men with spinal cord injury. Spinal Cord. 2001;39:208–214. doi: 10.1038/sj.sc.3101139. [DOI] [PubMed] [Google Scholar]
- WHO Study Group. Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis. Geneva, Switzerland: World Health Organization; 1994. [PubMed] [Google Scholar]
- McMahon K, Kalnius S, Reund J, et al. Discordance in lumbar T scores and non-standardization of standard deviations. J Clin Densitom. 2003;6:1–6. doi: 10.1385/jcd:6:1:1. [DOI] [PubMed] [Google Scholar]
- Faulkner KG, Roberts L, McClung M. Discrepancies in normative data between Luna & Hologic DXA systems. Osteoporos Int. 1996;6:432–436. doi: 10.1007/BF01629574. [DOI] [PubMed] [Google Scholar]